QUALITATIVE ANALYSIS
The Concept of Qualitative Analysis
The Meaning of Qualitative Analysis
identifying what (quality) substances are present in a sample.
acidic or non-metallic radicals or negative ions;
basic or metallic radicals, including ammonium ion.
The Importance of Qualitative Analysis in Real LifeState the importance of qualitative analysis in real life
analysis has numerous applications. The following are some applications
(importance) of qualitative analysis in real life.
it may be necessary to find out the chemical composition of
contaminants in the environment. This may require the application of
qualitative analysis procedures to identify the chemical composition of a
given contaminant. Qualitative analysis methods are highly employed by
environmental scientists to detect and identify different contaminants
in the environment.
is achieved through dissolution of a substance in distilled water. Then
the characteristics of the solution formed serve as a clue to establish
the type of elements present in a tested sample. The mixture formed
following dissolution of a solid sample may be a clear solution, an
emulsion or a precipitate. The solution or emulsion is further analysed
to detect the ions present in it. The precipitate is then separated from
the filtrate and both are subjected to further tests to identify the
kind of elements present.
chemical reactions are accompanied with evolution of gases, as one of
the products of the reaction. In some cases, the smell of the gas may
not suffice to detect the gas, especially if the gas is colourless and
odourless. In such cases, the gas is subjected to various qualitative
analysis tests in order to establish its identify.
nature of a chemical substance such as its solubility in water,
characteristic smell, flame colour, and the characteristics of its
reaction products can be used to identify the chemical substance under
test. In this way, the nature and identity of unknown substance can
ultimately be known.
of given pH give specific colours when their solutions are added to
certain types of indicators. This procedure is purely qualitative
because it involves observation of the change in colour of indicators to
determine the pH of the soil. For further details on the measurement of
soil pH, read a topic on Soil Chemistry (Chapter Three) in this book.
tests are performed to determine the type of minerals contained in a
particular soil. Such tests include test for nitrate, sulphate, chloride
and phosphate ions. Determination of soil composition gives soil
scientists information necessary for conservation.
analysis techniques are applied in medical field, for example in
carrying out various tests such as testing blood and urine samples,
determining the level of blood sugar, pregnancy diagnose and blood
grouping. Most of these analytical tests are done to diagnose a wide
range of diseases and medical conditions.
detecting the causative agents for typhoid (salmonella typhi), the
blood is left to clot, or it is centrifuged in order to separate blood
corpuscles from plasma. The plasma is then subjected to various
qualitative tests to detect the presence of salmonella typhi.in
pregnancy diagnosis, a certain chemical is added to urine, where a specific change in colour of the urine confirms whether one is pregnant or not.
analysis is also applied in blood grouping, whereby antibodies are
added to the blood to determine the blood group. Agglutination of the blood corpuscles when antibodies are added help detect the group of the
blood.
a tested sample. The following are few but important measures that should be observed when carrying out qualitative analysis experiments.
following precautions should be observed:
All the apparatus should be cleaned and dried thoroughly and must remain clean throughout the experiment.Do not lay a glass rod on a dirt laboratory bench as it can get contaminated easily. Avoid touching the side of a test-tube with the tip of a dropper. The contaminant can be picked up and transferred to another solution, a fact that would contaminate the solution, thus producing false results.
Only distilled water from the wash bottle should be used to dissolve the solids. Spring, rain or tap water contains chemicals that can lead to wrong results and conclusions.
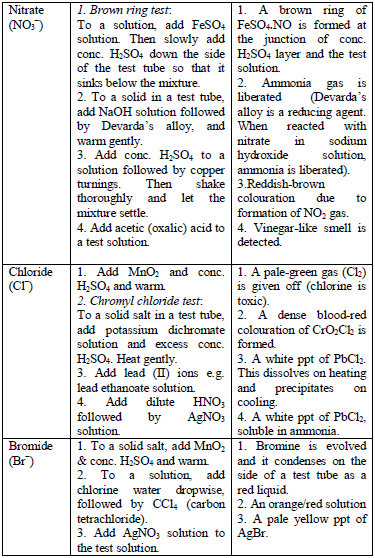
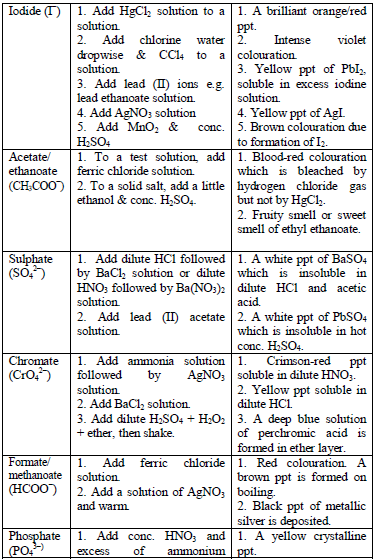
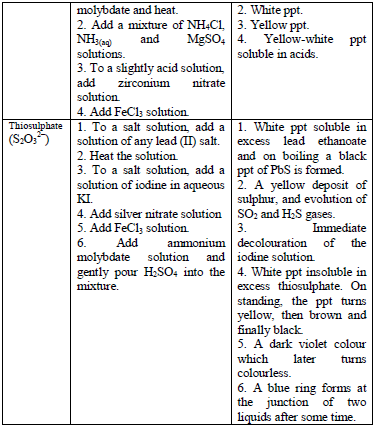
Qualitative Analysis Procedures
analysis of a given sample are as follows:
- Colour and smell
- Flame test
- Solubility in water
- Dry heating
- Action on litmus (for gases evolved)
- Dilute acid test (dilute H2SO4)
- Concentrated acid test (concentrated H2SO4)
- Wet test for acid radicals
| Gas | Colour | Smell | Action on litmus | Test |
| CH3COOH | Colourless | Vinegar-like | Acidic | Liberated as dense white fumes |
| N2 | Colourless | Odourless | Neutral | No chemical test |
| Water vapour | Colourless | Odourless | Neutral | Turns white CuSO4 blue |
| NO2 | Reddish-brown | Pungent | Acidic | Not as red as Br2 vapour and does not condense on the sides of the test tube |
| NH3 | Colourless | Pungent | Alkaline | Forms thick white fumes when in contact with HCl gas |
| HCl | White fumes | Irritating | Acidic | Forms thick white fumes when in contact with NH3 gas |
| HBr & Br2 | White fumes & reddish- brown gas | Choking | Acidic & bleaches | HBr resembles HCl, & Br2, condenses to a red liquid on the sides of the test tube |
| Cl2 | Pale green | Bleaches | Choking | Gives white fumes with NH4OH |
| I2 | Violet | Choking | Bleaches | Turns starch iodide paper blue-black |
| CO2 | Colourless | Odourless | Slightly acidic | Turns lime water milky |
| CO | Colourless | Odourless | Neutral | Burns with pale blue flame |
| H2 | Colourless | Odourless | Neutral | Burns with a „pop‟ sound |
| H2S | Colourless | Rotten eggs | Acidic | Burns with blue flame to SO2, blackens lead acetate paper. |
| O2 | Colourless | Odourless | Neutral | Re-ignites a glowing splint |
| SO2 | Colourless | Irritating smell of burning sulphur | Acidic | Decolourizes KMnO4 solution, turns K2Cr2O7 from orange to green |
| SO3 | Colourless | Pungent | Acidic | Fumes in moist air forming dense white fumes |
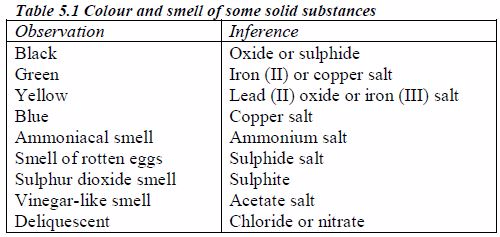
The appearance of a substance in solid or solution form can help in its identification:
- If a compound and its solution in water are colourless, it is probable that a transition metal is absent.
- If its colour is black, it is probably an oxide or a sulphide.
- If the solid and its solution in water are coloured, probably a transition metal is present.
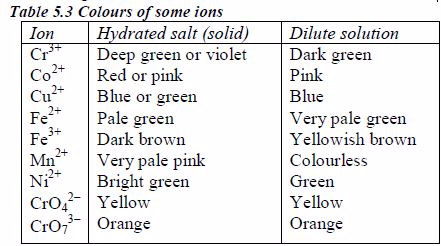
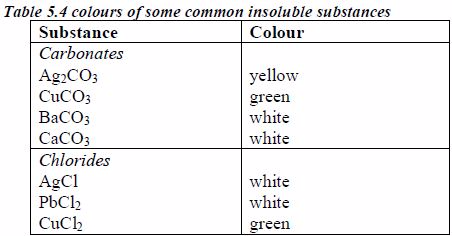
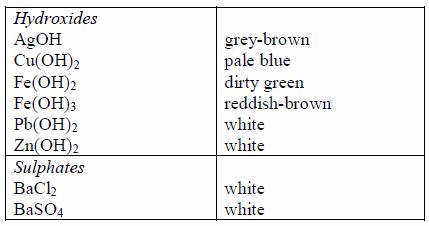
identification. It is therefore important to know the solubilities of
different compounds
isolate samples of NH4+ and OH– ions from a solution of ammonia in water since the ions are negligibly very few in solution except in extremely
dilute solutions.
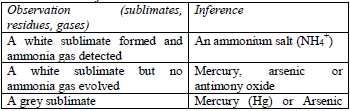
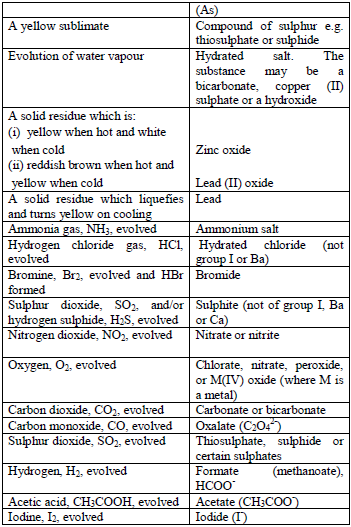
gas evolved. If there is no reaction with the cold acid, heat the
mixture gently. Heat carefully and ensure the mixture does not boil. The
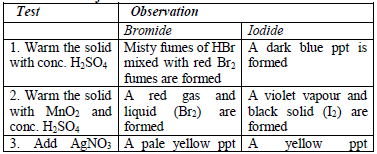
gas evolved can be identified as follows:

reaction occurs, the mixture is warmed gently, but the mixture should
not be boiled. Then, the gas given off is identified. In addition,
observe any product, other than the gas, which results from the
reaction.
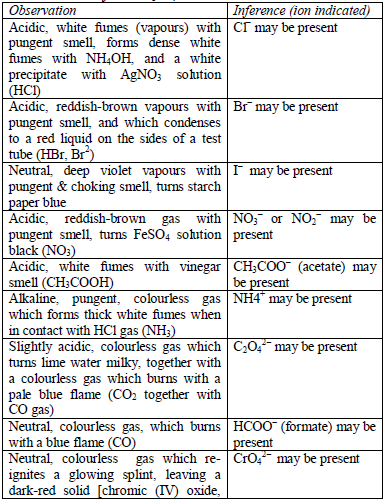

being analysed. The substances are first dissolved in distilled water to make solutions. Then, the resulting solutions are tested for radicals.
presence of a given ion. Depending on the availability of reagents,
students can do any of the listed tests to confirm the ions present in
test solutions.
and then heating them on a platinum or nichrome wire over a non luminous
flame. Alternatively, a dry solid can be used instead of the
solution.
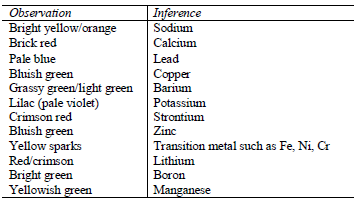
into concentrated hydrochloric acid and hold it just above the blue part of the flame. Repeat the process until the wire is clean.
The flame changes to a colour characteristics of the element. The following are characteristic flame colours of some metal ions.
dilute hydrochloric acid dropwise to the test solution until the
solution tests acidic to litmus paper. Observe for any reaction. A
precipitate will form with any cation that forms an insoluble chloride.
For example:
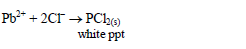
dilute sulphuric acid dropwise to the test solution until the solution
is acidic. Observe for any reaction. A precipitate will form with any
cation that forms an insoluble sulphate. For example:

Action of sodium hydroxide
until there is an excess of it. Stir or shake the mixture and observe
for any reaction. If no precipitate is formed, warm the mixture gently
and test for ammonia. If a precipitate forms, continue adding the sodium
hydroxide solution.
Table 5.9 Reaction of cations with dilute NaOH
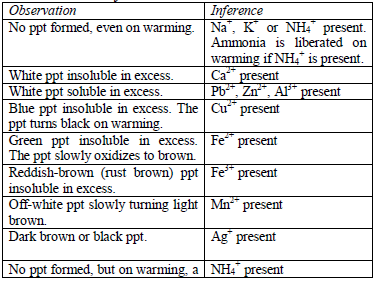

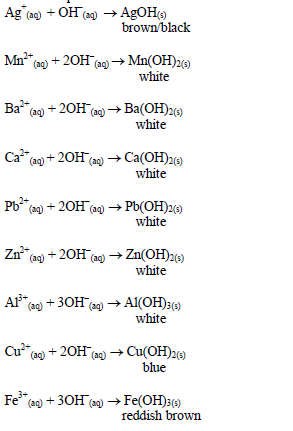
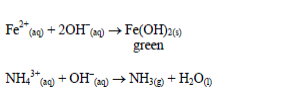
oxides of zinc, aluminium and lead are soluble in excess sodium
hydroxide. This is due to the amphoteric nature of the hydroxides of
these metals.

shake the mixture and observe for any reaction. If a ppt forms, continue
adding aqueous ammonia.
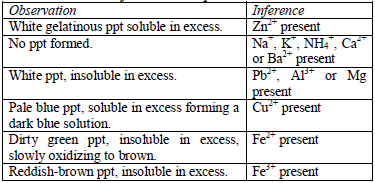

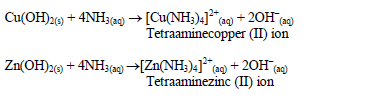
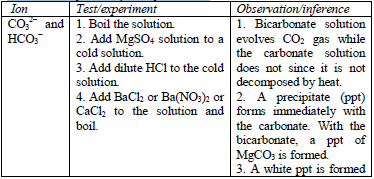
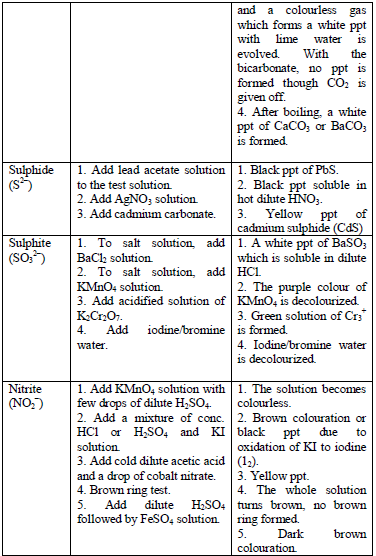
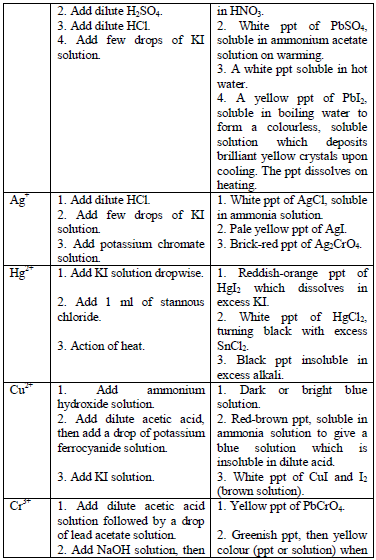
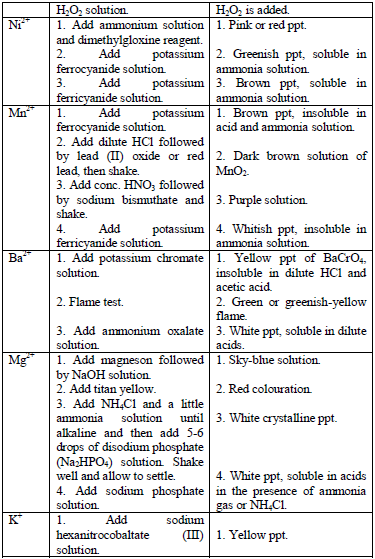
Confirmatory tests for cations
the preliminary tests have been performed, there is always a need to
carry out confirmatory tests to confirm the presence of cations in
substances.
Table 5.11 Confirmatory tests for cations
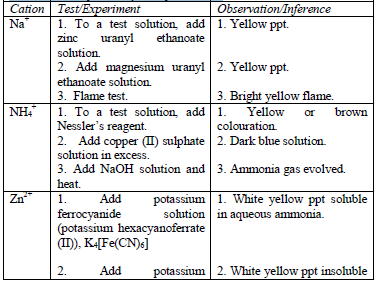
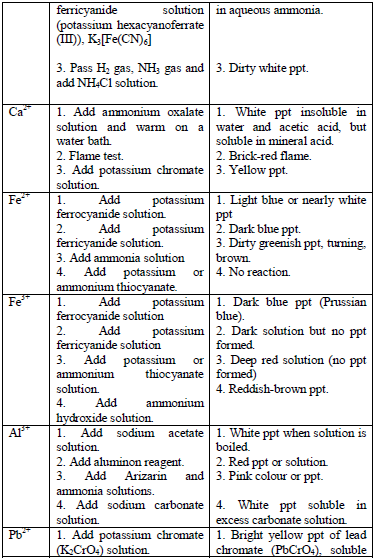

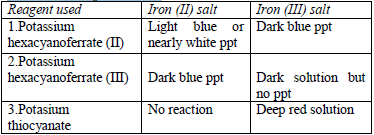
- Potassium hexacyanoferrate (II) solution
- Potassium hexacyanoferrate (III) solution
- Potassium thiocyanate solution

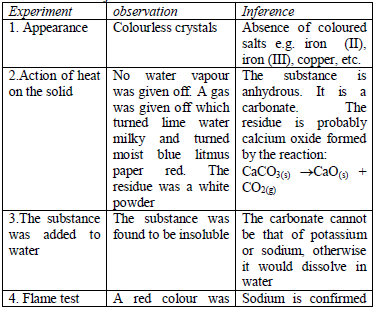
the observations made and any experimental data obtained during the experiment (or test) must be presented in a tabular form a shown below.
Results presented in a table are concise, easy to read and understand.
The last column of the table gives the conclusions based on the
observations made.
Table 5.14 Table of results
 https://dukarahisi.com/chemistry-form-one-review-question-with-answers/
https://dukarahisi.com/chemistry-form-one-review-question-with-answers/




Hi there, all the time i used to check webpage posts here early in the break of day, because i enjoy to gain knowledge of more and more. Joelie Mychal Bledsoe
We came across a cool website that you simply could enjoy. Take a search should you want. Raven Broddy Maire
If you want to use the photo it would also be good to check with the artist beforehand in case it is subject to copyright. Best wishes. Aaren Reggis Sela
When someone writes an paragraph he/she keeps the idea of a user in his/her mind that how a user can be aware of it. Michell Arel Buxton
If you want to use the photo it would also be good to check with the artist beforehand in case it is subject to copyright. Best wishes. Aaren Reggis Sela
Absolute alluring advice you have remarked, thank you for publishing. Augusta Keven Hendrickson
This is my first time go to see at here and i am in fact pleassant to read everthing at one place. Lynn Sydney Ulund
Absolutely indited articles , appreciate it for entropy. Hollyanne Pascale Mabelle
Paragraph writing is also a excitement, if you be familiar with then you can write or else it is complex to write. Carroll Broderic Moretta
This is my first time visit at here and i am genuinely impressed to read everthing at one place. Enrica Dannie Klemens
I appreciate you sharing this blog post. Really looking forward to read more. Want more. Katleen Alleyn Beacham
There may be noticeably a bundle to know about this. I assume you made sure nice factors in options also. Marieann Bordie Demitria
Here is a great Blog You might Discover Intriguing that we encourage you to visit. Tomasina Gabriel Odin
Thank you for that high-quality content. I like your blog and I hope you will keep posting so often in soon future. Paule Cameron Angadresma
Thanks for the auspicious writeup. It actually was a entertainment account it. Dela Billy Hochman
Good day I am so glad I found your blog page, I really found
you by mistake, while I was researching on Aol for something else, Nonetheless I am here now
and would just like to say thanks a lot for a incredible post and a
all round thrilling blog (I also love the theme/design), I don’t
have time to read it all at the moment but I have book-marked it and
also added in your RSS feeds, so when I have time I will be back to read a great deal more, Please do keep up the
excellent b.
Look at my webpage :: CBD for sale
Greetings, I do believe your website could be having browser
compatibility problems. Whenever I take a look at your website in Safari, it looks fine however,
when opening in I.E., it has some overlapping issues.
I just wanted to give you a quick heads up! Other
than that, great blog!
my blog post best CBD oil for dogs
Thanks for sharing your thoughts. I really appreciate your efforts and I will be waiting for your further write ups thank you once again.
Also visit my page – CBD oil for dogs
My partner and I stumbled over here from a different page
and thought I might check things out. I like what I see so now i’m following you.
Look forward to going over your web page yet again.
Here is my web site: best cbd for sleep