ATOMIC STRUCTURE
The Atom
We learned early that matter is made up of small, indivisible particles. Everything around us is made of extremely small particles. These particles are either molecules or atoms. An atom is the smallest indivisible particle of an element that takes part in a chemical change. Atoms are the building blocks of matter. All solids, liquids and gases are made of atoms fitted in different ways.
The present day chemistry is built on the foundations of the Atomic Theory. The idea that elements are made up of atoms is called the Atomic Theory. An English chemist, John Dalton was the first to put forward the Atomic Theory, which for most of the 19th century stated that atoms were hard, extremely small, indivisible and spherical particles like minute lead shots
Dalton Contribution to Atomic Structure
- Matter is made up of small, indivisible particles called atoms.
- Atoms of the same element are all exactly alike in every way and have definite weights.
- Atoms are indestructible and they cannot be created.
- Atoms of different elements have different weights and posses different properties.
- Atoms of different elements combine in small whole numbers to form ‘compound atoms’.
The Modern Concept of Dalton’s Atomic Structure
Some modifications to the theory include the following:
- The atom is no longer regarded as indivisible, or the smallest particle. Particles smaller than the atom; electrons, protons and neutrons are now known. However, the atom is still the smallest particle which can take part in a chemical reaction.
- Atoms of the same element may not be all alike. Some elements have atoms with different atomic masses e.g. carbon 12 and carbon 14. These different atoms of the same element are called isotopes.
- In some few cases, atoms of different elements may have the same atomic mass. Both argon and calcium have atomic mass 40. Such atoms are called isobars.
- “The compound atoms” of Dalton are known as molecules. A molecule is the simplest particle of matter which is capable of independent existence. Evidence is available where atoms of different elements combine in large integers. An example is in organic and silicon compounds.
- Atoms are no longer regarded as indestructible. Radioactive atoms may get destroyed by spontaneous decay or by atomic fission.The atom is therefore the smallest particle of an element which is responsible for the chemical properties of that element, and which takes part in a chemical reaction.
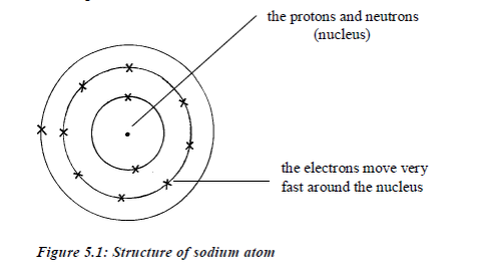
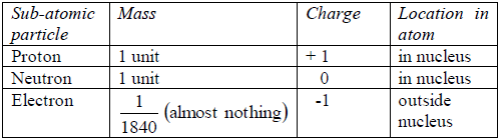
Electrons move around the nucleus in special paths calledelectron shells (orbits/or orbitals or energy levels). Protons andelectrons have electric charges. Neutrons have no charges.All the particles in an atom are very light. Their masses aremeasured in atomic mass units rather than grams.
The proton isa positively charged particle. Its mass is about equal to that ofhydrogen atom. The neutron is has no charge, it is neutral. Itsmass is about equal to that of hydrogen atom. The electron isnegatively charged. Its charge is equal but opposite to the chargeon the proton. It has a very small mass, about 1⁄1840 of the massof the proton.
The Properties of each Particle in an Atom
- Electrons are in orbit around the nucleus of the atom.
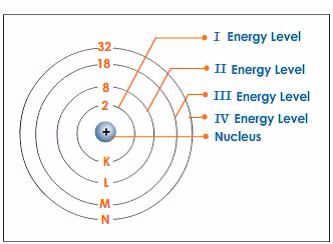
- The electron orbits are grouped together in shells; a shellis a group of orbits occupied by electrons withapproximately equal energy.
- The electrons in shells distant from the nucleus havehigher energy than those in shells close to the nucleus.
- Electrons fill the shells starting with the first shell, whichis closest to the nucleus. Shells are numbered 1, 2, 3, 4,98etc. outwards from the nucleus. The shells may berepresented by the letters K, L, M and N respectivelystarting from the nucleus.
- The maximum possible number of electrons in a shellnumbered n is 2 2n .
- The first shell can only contain up to 2 electrons. Thesecond shell can contain a maximum of 8 electrons. Thethird shell can contain up to 18 electrons.
- In the outermost shell of any atom, the maximumnumber of electrons possible is 8.
- The outer electrons of some atoms can be removed fairlyeasily to form ions.
- Chemical bonding between atoms to form moleculesinvolves the electrons in the outer shell only.
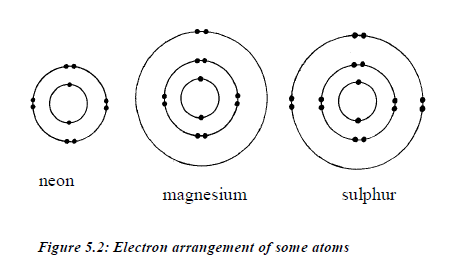
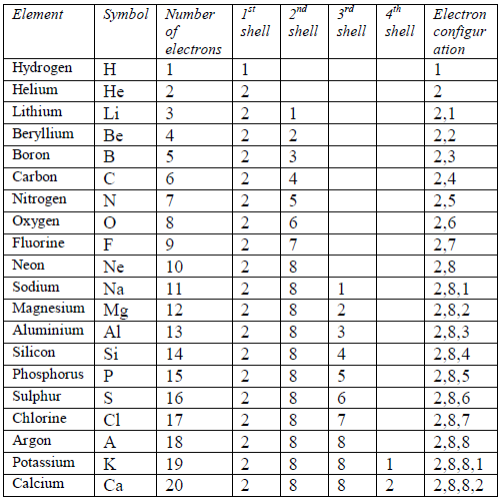
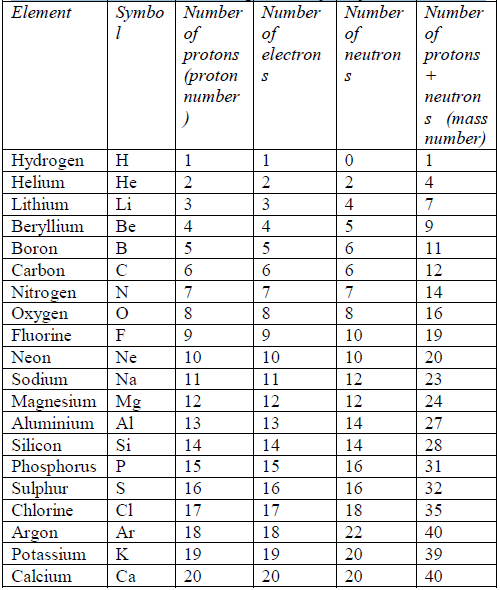
- Number of electrons = number of protons = atomic number
- Number of neutrons = mass number (A) – atomic number (Z).
Relative atomic masses