WATER
The Occurrence and Nature of Water
Describe the occurrence and nature of water
Water is the most abundant liquid in nature. It is a compound of hydrogen and oxygen. It occurs on land as seas, oceans, rivers, springs, wells, etc. It also occurs in the atmosphere as rain, water vapour, clouds, etc. Water is the essential constituent of animal and plant life. Without water, no life could exist on earth.
All living things need water to survive. About 60% of the human body by mass is made of water. A human being needs to drink about 2 litres of water per day to replace the water lost from the body via sweat, urine, breath, faeces, etc. If you did not replace this by eating and drinking, you would die in a matter of days.
Water is more important than food. A human being can survive without food for many weeks, but will die in a few days without water. So without water, no life can be sustained.
Water is the main constituent of the earth’s surface. 70% of the earth’s surface is covered by water. The remaining 30% is covered by land.
There are four kinds of natural water namely, rain water, spring and well water, river water, and lake and sea water. Natural water is never pure. Water from difference natural sources contains substances dissolved in it.
Rain water
This is naturally distilled water. It is almost pure and it contains only gases and dust dissolved from the air. If the dissolved gases are acidic, e.g. sulphur dioxide, carbon dioxide or nitrogen dioxide, they may form “acid rain”.
In heavily industrialized countries where emission of these gases is very great, acid rains have been experienced. Rain water in non-industrial areas is fairly pure. It is safe to drink though it is tasteless. The taste in water is due to dissolved substances in it.
When the rain falls, some water sinks into the ground to form ground water. This water percolates down the earth until it meets layers of impervious or impermeable (non-porous) rocks, which stop it from percolating or seeping any further. The ground water may reach the earth’s surface as a spring. When a whole deep enough is dug to reach the ground water, a well results. Spring or well water is supposed to be clean, although it contains dissolved substances. As water passes through the earth, it is naturally filtered.
River water
River water contains dissolved and suspended solid materials. The water in some rivers is very muddy or sandy depending on the nature of the land from which the river originates and on which it flows. Most of the water we drink or use at home and industries is from rivers. To make the river water fit for use, all the substances dissolved and suspended in it must be removed or filtered.
Lake and sea water
Lakes and seas receive water from rivers. River water contains dissolved salts. As it flows through the land, some of its water evaporates into the air. When it reaches the sea or lake, more water still evaporates. As a result, sea and lake water will necessarily contain vast quantities of dissolved substances.
Sea water contains about 3.6% by mass of the dissolved solids. Most of the dissolved solids compose largely of sodium chloride that can be obtained from sea water in large quantities. Three quarters of the ocean salts is sodium chloride (common salt).
The Water Cycle
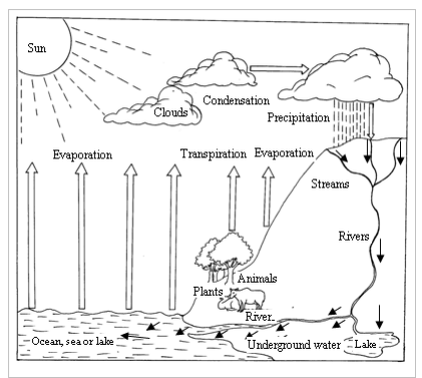
Water is always on move, travelling a never-ending, cyclical journey between the earth and the sky. This journey is referred to as the water cycle or hydrological cycle. The water cycle describes the continuous movement of water on, above and below the surface of the earth.
During its movement, water is continuously reused and recycled. It also changes its physical state or form (liquid, vapour, and ice) at various stages in the water cycle. Figure 3.1 is a diagrammatic representation of the water cycle. It shows how the water moves around the earth’s environment, changing its form through the process of evaporation, transpiration (loss of water from plants), condensation and precipitation (rainfall, snow, hail, fog, smog, etc.)
Stages of the water cycle are described below:
- Heat from the sun causes water to evaporate from exposed water bodies such as oceans, seas, lakes, rivers dams, etc. This causes huge amounts of water vapour to float (laden) in the air. The vapour rises up. In the cooler upper parts of the atmosphere, the vapour cools and condenses to form tiny water droplets. The droplets form clouds.
- The clouds are drifted by wind. They cool further, and the droplets join to form larger drops of water which fall down as rain due to gravitation pull. On the other hand, if the air is very cold, they fall as hail, sleet or snow. The whole process is called precipitation.
- Some rain water soaks, and reappears as springs. Some flows over the ground as streams. The springs and streams feed rivers. The rivers flow to the ocean, sea or lake. The whole cycle starts again.
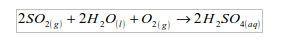
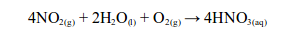
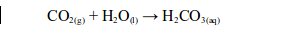
Includes
- Extremely pure water is colourless, odourless and tasteless. The colour, taste or odours in water are due to dissolved impurities of organic and inorganic nature.
- Pure water is a very poor conductor of heat and electricity. However, water containing some dissolved inorganic impurities may conduct appreciably.
- Pure water freezes at 0ºC.
- Pure water boils at 100ºC at a pressure of 760 mmHg; and pure water will boil away completely with no change in temperature. Its melting point and boiling point are abnormally high due to hydrogen bonding.
- It is the only substance that occurs naturally in all the three states of matter – solid, liquid and gas.
- Water, as compared to other liquids, dissolves almost all substances, though in varying degrees of solubility. For this reason, water is usually called the universal solvent.
- It has a high surface tension than other liquids.
- It has a high specific heat index, which means that it can absorb a lot of heat before getting hot.
- It is miscible with many liquids, for example ethanol.
- The maximum density of pure water is 1 g cm-3 at 4ºC. When water is cooled gradually, it reaches its maximum density at 4 centigrade. The actual change from water to ice takes place at 0ºC.
- Pure water is neutral to litmus and has a pH of 7.0.
- Water expands when it freezes. Most substances contract when they change from liquid to solid state. Water is one of the very few substances that expand when they freeze. This behaviour is called anomalous expansion of water. Ice is therefore much less dense than water. The water molecules in the ice crystals are further apart from each other than in liquid water.
Chemical properties

Potassium
Potassium is vigorously attacked with cold water, producing hydrogen gas. The reaction of water with potassium is very violent and the hydrogen produced catches fire spontaneously with a lilac flame. The colour is due to the burning of small quantities of potassium vapour.
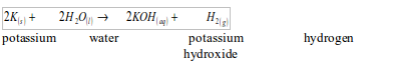
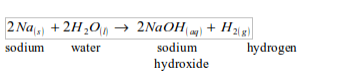
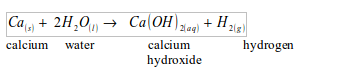

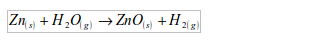
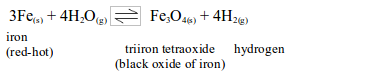
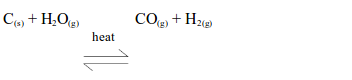

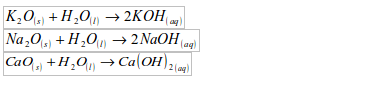

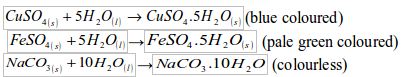
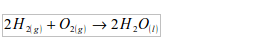
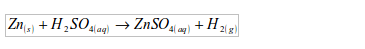
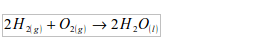
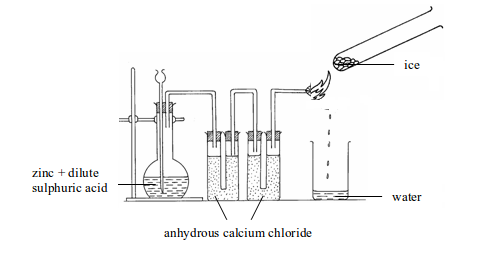
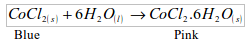
- Will turn anhydrous copper (II) sulphate from white to blue.
- Will turn anhydrous cobalt (II) chloride from blue to pink.
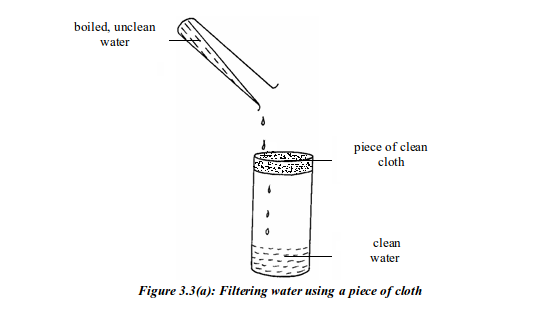
- bacteria – most are harmless, but some can cause diseases.
- dissolved substances – for example, calcium and magnesium compounds dissolved from rocks; and gases from the air.
- solid substances and debris – particles of mud, sand, grit, twigs, dead plants and perhaps tins and rags that people have dumped.
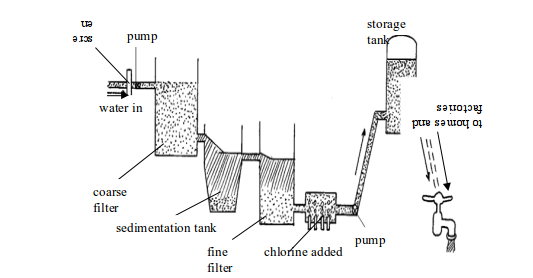
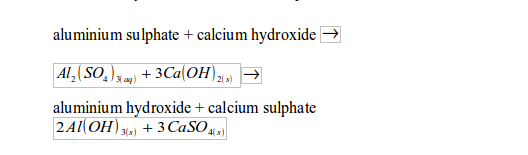
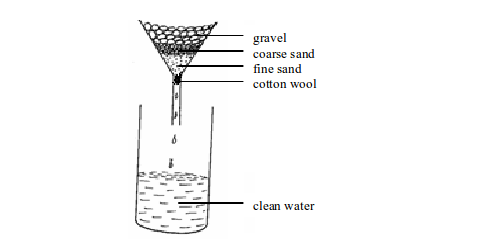
- To kill harmful and disease-causing microorganisms such as bacteria, fungi, actinomycetes, amoeba, salmonella, etc.
- To remove toxic substances dissolved in water
- To remove solid substances and debris from the water such as tins, lags, plant remains, sand, algae, spirogyra, etc.
- To remove suspended earthy material (clay matter)
- To remove odour and unpleasant smells caused by different contaminants dissolved in water.
- To remove water hardness – sodium carbonate is added in water to remove both temporary and permanent hardness in water to make the water soft. Soft water forms lather easily with soap as compared to hard water which forms scum instead. This means that soft water requires less soap to form enough lather than hard water does. Therefore, soft water saves soap and hence money that could have been spent to purchase extra soap for washing.
- The sodium fluoride added to water in some areas helps to fight tooth decay.
Water has numerous uses in life. The following are some of the uses of water:
Biological use: Water is essential to life. Most of the reactions in animals and plants take place in solutions in water. Plants absorb minerals from the soil in solution form. Animals and plants are found near or in areas where water can be found.
Domestic use: Domestic water use is probably the most important daily use of water for most people. It includes water that is used in the home every day including water for normal household purposes such as washing clothes and dishes, drinking, bathing, food preparation, flushing toilets, and watering lawns and gardens, etc.
Industrial use: Water is a valuable resource to the nation’s industries for such purposes as processing, cleaning, transportation, dilution, and cooling in manufacturing industries. Major water-using industries include cloth, steel, chemical, paper, and petroleum refining. Industries often reuse the same water repeatedly for more than one purpose. Water is used as a solvent in many industrial processes. It is also used for cooling certain parts of machines.
Irrigation: Water is artificially applied to farm, orchard pasture, and horticultural crops, as well as leaching of salts from the crop root zone in sodic soils. Non-agricultural activities include self-supplied water to irrigate public and private flower gardens, loans, football pitches, etc. Crop production in areas that receive little rainfall per year can be achieved through the practice of irrigation. Water for irrigation purposes can be drawn from rivers, lakes, swamps and even from seas.
Water as a solvent: Water is regarded as a universal solvent. It dissolves almost all substances. For this reason, it is used for dissolution of chemicals ranging from poisonous chemicals used in agriculture to non-poisonous chemicals used in hospitals, laboratories, research stations and for other general purposes.
Cooling and heating: Due to its high specific heat capacity, water is used as a coolant for cooling automobile engines and other machines. Hot water is used during winter for heating homes in temperate countries. In higher plants, evaporation causes a cooling effect and therefore helps to cool plant organs. During hot weather, some animals tend to wallow in water in order to cool their bodies either through evaporation or by water itself.
Habitat: Water is a habitat for fish and all aquatic animals and plants.
Livestock use: This includes water for stock animals, feedlots, dairies, fish farms and other non-farm animals. In arid regions of Tanzania, the Government has constructed dams to supply water to cattle, and for some domestic uses.
Mining: Water is used in mines for extraction of naturally occurring minerals: solids, such as coal and ores; liquids, such as crude petroleum; and gases, such as natural gas. This includes quarrying, milling (such as crushing, screening, washing, and flotation), and other operations as part of mining activity.
Generation of electricity: Hydroelectric power is generated by river water. Fast-moving river water (especially in waterfalls and cataracts) is used to turn turbines to generate hydroelectricity that is supplied to homes, industries, towns, etc. Most of the electricity we use at home is generated by this means. Only a small portion is generated through other means.
Navigation and recreation: People, goods and services can be transported via water bodies like rivers, lakes and oceans by using vessels such as boats, dhows, canoes and ships. Water is also used for sports such as swimming, canoeing, fishing, yachting, water skiing, and many other sports carried out on, in and under the water.
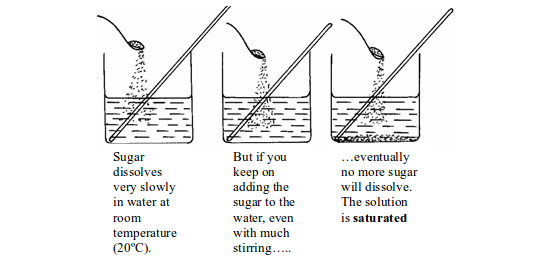
The Solubility of Different Substances in Water and Organic Solvents
Water is the commonest solvent in use, but other liquids, are also important. The other solvents are generally organic liquids such as ethanol, propanone, trichloroethane, etc. These organic solvents are also important because they will often dissolve substances that do not dissolve in water. The following table shows an example of substances that dissolve in water.
Substances soluble and insoluble in water
| Soluble compounds | Insoluble compounds |
| 1 All common sodium, potassium and ammonium salts | |
| 2. All common nitrates of metals | |
| 3. All common chlorides except………………….. | silver, mercury (I) and lead chloride |
| 4. All common sulphates except………………….. | lead, barium and calcium sulphates |
| 5. Sodium, potassium, and ammonium carbonates… | but other common carbonates are insoluble |
| 6. Sodium, potassium and ammonium hydroxides… | but other common hydroxides are insoluble. |
| Compound | Mass (g) dissolving in 100g of water at 25ºC |
| Silver nitrate | 241.3 |
| Calcium nitrate | 102.1 |
| Magnesium chloride | 53.0 |
| Potassium nitrate | 37.9 |
| Potassium sulphate | 12.0 |
| Calcium hydroxide | 0.113 |
| Calcium carbonate | 0.0013 |
| Silver chloride | 0.0002 |
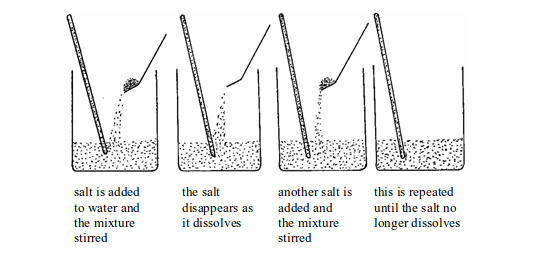
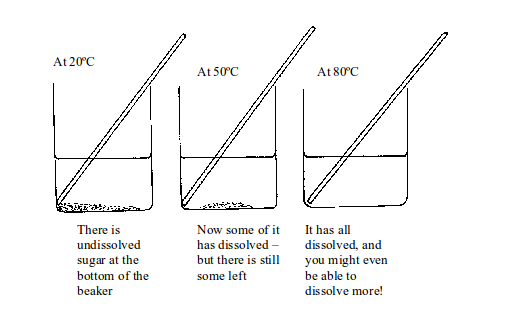
- Put a weighed amount (say 2g) of potassium sulphate in a test tube. Add a little water from a measuring cylinder.
- Heat the test tube gently until the water is hot but not boiling. Add more water if necessary until the solid is just dissolved.
- Let the solution cool while stirring it with a thermometer. Note the temperature at which the first crystals form.


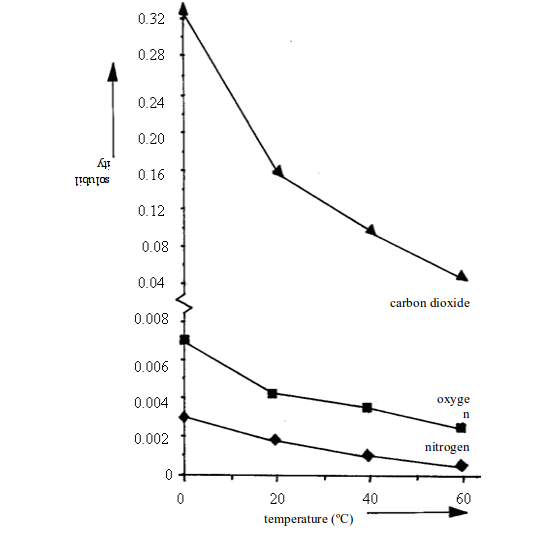
| Gas | Solubility (cm3 per 100cm3 of water) at….. | |||
| 0ºC | 20ºC | 40ºC | 60ºC | |
| Oxygen Carbon dioxide Sulphur dioxideHydrogen chloride | 4.8171798050500 | 3.392.3425047400 | 2.556.6217044500 | 1.936.0-42000 |
| Temperature in ºC | Solubility in g of salt per 100g of water | ||
| Sodium chloride | Copper (II) sulphate | Potassium nitrate | |
| 10 | 38 | 18 | 20 |
| 20 | 38 | 20 | 30 |
| 30 | 38 | 24 | 44 |
| 40 | 38.5 | 28 | 60 |
| 50 | 38.5 | 34 | 80 |
| 60 | 39 | 42 | 104 |
| 70 | 39 | 50 | 152 |
- Which of the salts is the most soluble at 15ºC?
- Which of the three salts is the most soluble at 55ºC?
- At which temperature do sodium chloride and potassium nitrate have the same solubility?
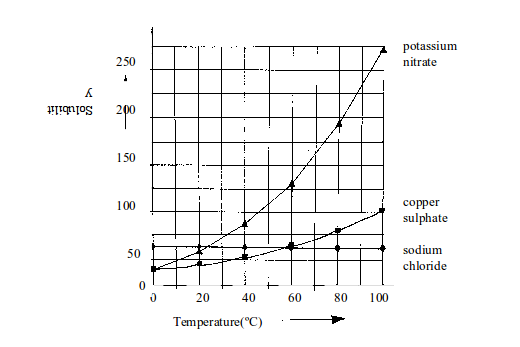
- 40ºC and
- 50ºC?
- At 50ºC, 137.5g of potassium nitrate dissolve in 100g of water.
- At 40ºC, 62.5g of potassium nitrate dissolve in 100g of water.




















Leave a Reply