acids. For example, orange juice and grapefruit juice contain citric
acid. These juices, and others of the like, contain ascorbic acid, a
substance more commonly known as vitamin C. Examples of natural sources of acids and the type of acids they contain are shown in table below.
| Source | Type of acid present |
| Mineral acids (HCl, H2SO4, HNO3, etc.) | Minerals |
| Tobacco | Salicylic acid |
| Tea | Tannic acid |
| Coffee | Chlorogenic acid |
| Sugar beet | Glutaric and adipic acids |
| Blackberry | Isocitric acid |
| Spinach, tomato | Oxalic acid |
| Sour (fermented milk) | Lactic acid |
| Bee, ant and nettle stings | Methanoic acid (formic acid) |
| Grapes, bananas, tamarinds | Tartaric acid |
| Citrus fruits | Citric acid ( lemons and limes have particularly high concentrations of citric acid; it can constitute as much as 8% of the dry weight of these fruits) |
fermented milk are all sour tasting because of the presence of acids.
The acids present in animal and plant materials are known as organic acids.
Boric acid is a substance that is sometimes used to wash the eyes.
naturally occurring compounds called minerals. Mineral acids are
generally stronger and should be handled with great care, especially the
concentrated acids, for they are very corrosive. They can eat away
metals, skin and clothing. Nevertheless, some acids are not corrosive
even when they are concentrated. They are called weak acids. Ethanoic
acid is one example. It is found in vinegar. In general, organic acids
are weaker than natural acids.
is a purple dye. It can be used as a solution, or on paper, called
litmus paper.Litmus solution is purple. Litmus paper for testing acids
is blue while that for testing bases is red in colour. Acids will turn
litmus solution red. They will also turn blue litmus paper red.
industry. Bases can be classified into oxides, hydroxides or carbonates.
Therefore, bases can be defined as the oxides, hydroxides or carbonates
of metals. Bases taste bitter. A bitter taste is a characteristic of
all bases.
different indicators. We can use this clue of colour changes to tell
whether an unknown substance is an acid or base (alkali).
| Indicator | Colour in acid | Colour in alkali (base) |
| Methyl orange | orange | yellow |
| Phenolphthalein | colourless | Pink |
| Litmus | red | blue |
| Bromothymol blue | yellow | blue |
reactions are best carried out by using dilute acid solutions. The
following are some reactions of dilute acids with various substances.
Mg(s) + 2HNO3(aq) → Mg(NO3)2(aq) + H2(g)
Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
react with carbonates to give salt, water and carbon dioxide. In
general, all carbonates give off carbon dioxide when they react with
acids.
normal methods of preparing carbon dioxide in the laboratory are based
on this reaction. Dilute hydrochloric acid is reacted with marble chips
(calcium carbonates):
bases (oxides, hydroxides) all react in the same way with acids, and in
the process, salts are formed. This type of reaction is known as neutralization reaction. It can be summarized up in a general equation:
react with acids to produce salt and water. All alkalis, except ammonia
solution, will react with ammonium compounds liberating ammonia gas.
Aqueous solutions of alkalis will precipitate the insoluble hydroxides
of other metals from the solutions of metal salts. Caustic alkalis
attack aluminium, zinc and lead to form salts. They react with carbon
dioxide to form carbonates.
Table 3.2 shows how acids and bases (alkalis) affect the colours of
different indicators. We can use this clue of colour changes to tell
whether an unknown substance is an acid or base (alkali).
solutions of alkalis will precipitate the insoluble hydroxides of other
metals from the solutions of metal salts. Only NH4OH, KOH and NaOH are soluble enough in water.
When
sodium hydroxide solution is added to copper (II) sulphate solution, a
pale blue precipitate of copper (II) hydroxide is formed. CuSO4(aq) + 2NaOH(aq) → Cu(OH)2(s) + Na2 SO4(aq)
Another example is the reaction between potassium hydroxide and iron (II) chloride, which precipitates iron (II) hydroxide. FeCl3(aq) + 3KOH(aq) → Fe(OH)3(s) + 3KCl(aq)
alkalis attack very few metals. The metals known to be attacked by the
alkalis are aluminum, zinc and lead, where the aluminate, zincate and
plumbate (II) are formed respectively. The aluminum will react thus:2Al(s) + 6NaOH(aq) + 6H2O(l) → 2Na3Al(OH)6(aq) + 3H2(g)(sodium aluminate)
dilute hydrochloric acid produced in your stomach is used for digestion
and killing bacteria that might have been swallowed together with food
or taken with water. However, excess acid causes indigestion, which can
be painful. To ease the pain, we take an anti-acid treatment. Anti-acids
are a broad group of compounds with no toxic effects on the body. They
are used to neutralize the effects of acid indigestion.
This treatment, therefore, prevents indigestion and pains. The
neutralization reaction equation is:
anti-acids such as “Alka-Seltzer” contain soluble materials, including
sodium hydrogencarbonate. These tablets also contain some citric acid (a
solid acid). On adding water, the acid and some of the sodium
hydrogencarbonate react, producing carbon dioxide gas. This helps to
spread and dissolve the other less soluble material. When taken, more
sodium hydrogencarbonate neutralizes the excess hydrochloric acid in the
stomach, thus easing digestion.
anti-acid tablets also contain painkiller to relieve pain. “Soluble
aspirin” tablets dissolve and work in a similar way to “Alker-Seltzer”
tablets. Vitamin C (ascorbic acid) can be added to the tablets. Note
that it is important to add water to start the action of the acid.
is formed inside boilers, kettles and water heaters when hard water is
boiled. The limescale can be removed by treatment with an acid that is
strong enough to react with CaCO3, but not strong enough to
damage the metal. Vinegar can be used to discale kettles. Commercial
“discalers” use other acid solutions such as methanoic acid
remnants sticking onto teeth (plaque), after eating especially sugary
food is acted upon by bacteria in your mouth. The pH of a sugar solution
is 7. However, bacteria in your mouth break down the sugar in plaque to
form acids, for example lactic acid. These acids lower the pH. Tooth
decay begins when the pH falls below 5.8. The acid attacks the tooth
enamel.
help prevent tooth decay many types of toothpaste contain basic
substances to neutralize the acids produced by these bacteria in your
mouth. The pH of these basic substances is alkaline (higher than 7). The
pH of saliva is slightly alkaline (pH 7.4), so it can also help to
counteract the acid, particularly after a meal. After eating a sweat,
for example, it takes about 15 minutes for saliva to raise the pH above
5.8, and stop further decay.
plants grow best when pH of the soil is close to 7. They prefer the pH
of between 6.5 and 7.0. If the soil pH is below 6.0, the soil is too
acidic. Above the pH of 8.0, the soil is too alkaline. If the soil is
too acidic or too alkaline, the plants grow poorly or not at all.
can be added to the soil to adjust its pH. Most often, if the soil is
too acidic, it is usually treated by liming. In this context, liming
means addition of quicklime (calcium oxide), slaked lime (calcium
hydroxide) or powdered chalk or limestone (calcium carbonate) to an
acidic soil. These compounds (bases) have the effect of neutralizing the
acidity of the soil.
the soil is too alkaline, acids such as sulphuric acid, nitric acid or
hydrochloric acid may be added to the soil to neutralize excessive
alkalinity. However, these compounds are very expensive and hence
uneconomical to apply on large-scale basis.
a bee stings someone, it injects an acid liquid into the skin. The bee
sting, which is acidic in nature, can be neutralized by rubbing on calamine solution,
which contains zinc carbonate or baking soda, which is sodium
hydrogencarbonate. These compounds are basic in nature and so have the
effect of neutralizing the acid in the sting.
stings are alkaline in nature, and can be neutralized with vinegar,
which contains ethanoic acid. Ant and nettle stings contain methanoic
acid. These may be neutralized by rubbing an extract squeezed from
crushed onion leaves (which contain basic compounds) on the affected
skin. The acid in the sting can also be neutralized by applying weak
alkalis such as ammonia solution, ash extract, baking powder, etc.
wastes from factories often contain acid. If it reaches a river, lake
or ocean, the acid will kill fish and other aquatic life. This can be
prevented by adding slaked lime (calcium hydroxide) to the waste, to
neutralize the acid before being dumped into water bodies.
coloured substances (many extracted from plants) have been found to
change colour if added to an acid or alkaline solution. The colour
change is reversed if the acid or alkali is neutralized. Substances that
behave like this are known as indicators.
Changing this red colour of litmus needs a chemical reaction. The
molecules of the indicator are usually changed in the presence of the
acid. Substances with the opposite chemical effect to acids are needed
to reverse the change, and these are called alkalis. They turn litmus
solution to blue. Litmus can also be used in paper form, in which case it is called litmus paper. Here it comes in the blue and red forms. Litmus is a single chemical compound. It gives a single colour change.
These indicators give different colour changes when in acidic and
alkaline solutions (see table 3.2).Another commonly used indicator is
the universal indicator (or full-range indicator). This is made
from a mixture of dyes. Such an indicator is useful because it gives a
range of colours (“spectrum”) depending on the strength of the acid or
alkali added (see table 3.3)
a universal indicator, different acids produce a range of different
colours. Indeed, solutions of the same acid with different
concentrations (pH) give different colours.
learned that many indicators are extracted from plants. Flowers and
leaves of different plants have different colours. These plant organs
may be used to prepare indicators locally.
Collect flowers from different plants in your local area. You may use coloured leaves if the coloured flowers are not available.
Crush the flowers/leaves in a motor and pestle to make a fine paste.
Add ethanol to the paste to wash out chlorophyll. Add about 10cm3 of ethanol per gram of pestle used.
Grind the mixture to a very fine paste so that the ethanol can penetrate the broken plant cells fully.
Place the mixture in the sun or heat gently to evaporate off ethanol. Make sure most of the ethanol has evaporated.
Filter
the mixture to obtain a clear but coloured filtrate. To obtain as much
extract as possible, squeeze the paste in a clean piece of cloth and
collect the juice in a beaker. The liquid you obtain is your indicator.
Arrange test tubes in a rack and label them A, B, C D and E.
Pour
sodium hydroxide, dilute hydrochloric acid, limewater, lemon juice,
vinegar and washing soda in test tubes A, B, C, D and E respectively.
Add two to three drops of the prepared indicator in each of the test tubes. Observe and record the colour changes.
What was the colour of your indicator?
Write down the colour changes in each of the test tubes A to E.
Which substance showed a sharp colour change?
Perform
a similar experiment using a ready-made universal indicator and observe
whether there is any difference in colour changes between this
commercial indicator and that one prepared from local plants.
is a big difference between the strength of an acid or base and its
concentration. An acid or alkaline solution is said to be concentrated
if it contains a large amount of it in a small amount of water. A dilute
acid or base (alkali) has a small amount of it in a lot of water. The
concentration of an acid or base tells us how much of it is dissolved in
a certain volume of solution. The concentration is normally expressed
in grams per litre (g dm-3) or moles per litre (mol dm-3).
strength of an acid or alkali expresses its dissociation in water.
Strong acids or alkalis will dissociate completely in water to form
ions. Examples of strong acids are sulphuric acid, hydrochloric acid,
nitric acid and phosphoric acid. Weak acids include ethanoic acid,
carbonic acid and methanoic acid. Examples of strong alkalis include
potassium hydroxide, sodium hydroxide, calcium hydroxide and ammonium
hydroxide. Weak bases include ammonia solution and sodium
hydrogencarbonate.
formed when it dissociates in water, determines the strength of an
acid. The strength of an alkali depends on the number of hydroxyl ions,
OH–, formed when it dissociates in water. Strong acids and alkalis will form many H+ and OH– ions respectively. Weak acids or bases will form very few of the respective ions.
have seen that single indicators change their colours only once when
put in different acid and alkaline solutions. The single indicators most
commonly used include litmus, phenolphthalein and methyl orange.
indicators can only tell us whether a certain solution is an acid or an
alkali. These types of indicators cannot be used to compare two acids
or two alkalis with different strengths. Litmus paper, for example,
cannot be used to compare the strengths of sulphuric acid and ethanoic
acid. Both acids will change the blue litmus paper to red. Likewise, you cannot compare the strengths of aqueous ammonia solution (NH4OH) and sodium hydroxide by just using a litmus paper. They will both turn to red litmus paper to blue.
universal indicator can be used to measure strengths of different acids
and alkalis. This indicator is a mixture of simple indicators. Instead
of changing colour just once, it changes colour a number of times
depending on the degree of acidity or alkalinity of the substances
tested.
pH scale is a convenient means of expressing the acidity and alkalinity
in liquids. The pH scale is a numerical scale used to indicate the
relative strengths of acidic or basic solutions in terms of relative
amount of hydrogen ions (protons) or hydroxyl ions in solutions. The
scale ranges from 0 to 14.
solutions will have pH values less than 7.0 and alkaline solutions will
have pH values greater then 7.0. All neutral liquids e.g. pure water
have pH of 7.0. Table 3.3 shows the pH and strengths of acidic and
alkaline solutions and the associated indicator colour changes.
| pH range | Colour | Strength |
| 1, 2, 3 | Red | Strongly acidic |
| 4 | Orange | |
| 5, 6 | Yellow | Weakly acidic |
| 7 | Green | Neutral |
| 8, 9 | Blue Indigo | Weakly alkaline |
| 10, 11, 12, 13, 14 | Purple/violet | Strongly alkaline |
that there is no clear dividing line between the pH ranges as
apparently shown in the above table. This means that you may have
substances with, for example, pH 1.2, 1.5, 3.5, 4.4, 5.6, 8.4, etc. The
table just tries to simplify the concept of acidity and alkalinity of
acid and alkaline solutions.
salt is a substance formed when some or all of the hydrogen atoms of an
acid are replaced by a metal or ammonium ion. A salt, therefore, may be
defined as a compound in which the replaceable hydrogen of an acid has been wholly or partially replaced by a metal.
sodium chloride (NaCl), for example, the hydrogen atom of hydrochloric
acid (HCl) has been wholly replaced by an atom of sodium. In magnesium
sulphate (MgSO4) and sodium sulphate (Na2SO4), both hydrogen atoms of sulphuric acid (H2SO4) have been replaced by one atom of magnesium and two atoms of sodium respectively. In sodium hydrogen sulphate (NaHSO4),
only one out of two hydrogen atoms has been replaced by an atom of
sodium. This type of a salt is called an acid salt, because it still
contains a replaceable hydrogen atom.
chemical compounds may be classified as salts. The salt most familiar
to every body is table salt (sodium chloride). Baking soda is the salt,
sodium bicarbonate (NaHCO3). Magnesium sulphate (also called Epsom salt) is often found in the home.
general, salts are ionic impounds that are composed of metal and non
metal ions. For example, sodium chloride is is composed of metallic
sodium ions (Na+) and non-metallic chloride ions (Cl–). Some salts are made of metallic and non-metallic radicals e.g ammonium nitrate (NH4NO3) is composed of ammonium radical (NH4+) and nitrate radical (NO3–).
is a wide range of types and natural sources of salts. Common salt is
mined from underground deposits. The salt obtained from such a source
contains sodium chloride mixed with rock impurities.
other source of sodium chloride is seawater. The salty taste of
seawater is due to the presence of salts such as sodium chloride and
magnesium bromide. However, there are many different types of salts
present in seawater, though in small proportions, as shown in the table
below (table 3.5)
| Salt | Formula | Percentage composition |
| Sodium chloride | NaCl | 2.72 |
| Magnesium chloride | MgCl2 | 0.38 |
| Magnesium sulphate | MgSO4 | 0.17 |
| Calcium sulphate | CaSO4 | 0.13 |
| Potassium chloride | KCl | 0.09 |
| Calcium chloride | CaCO3 | 0.01 |
| Magnesium bromide | MgBr2 | 0.01 |
are found in underground deposits. Calcium carbonate occurs naturally
as marble, limestone or chalk in the ground from which it can be mined
mechanically. What other natural sources of salts do you know?
may be classified according to their mode of formation. The following
are types of salts grouped according to their mode of formation:
This is a salt formed when all of the replaceable hydrogen atoms of an
acid have been replaced by a metal atom e.g. sodium chloride is a normal
salt because all hydrogen atoms are replaced from an acid during its
formation.
An acid salt is a salt formed when part of the replaceable hydrogen
atoms of an acid are displaced by a metal e.g., sodium bisulphate (NaHSO4) is an acid salt.
they react with bases to form salts and water only. NaHSO4(aq) + NaOH(aq)→ Na2SO4(aq) + H2O(l)
they react with carbonates to yield carbon dioxide.2NaHSO4(aq) + Na2CO3(aq)→ 2Na2SO4(aq) + H2O(l) + CO2(g)
A basic salt is formed by the action of an acid with higher proportions
of the base, than is necessary for the formation of a normal salt.
basic salt may also be formed by the partial replacement of the
hydroxyl groups of a diacidic or triacidic base by an acid radical.
salts are usually insoluble in water. Such salts are formed by the
close association of two simple salts, when crystallized from a solution
of a mixture of the two.
salts are more soluble in water than others are. However, other salts
are insoluble in water. The knowledge of solubility of different salts
in water is very important because it can help us prepare different
salts in the laboratory by such methods as precipitation, direct
combination (synthesis), crystallization and so forth.
regards to solubilities, salts can be classified into two groups: salts
which are soluble in water (soluble salts) and salts which do not
dissolve in water (insoluble salts). Table 3.6 summarizes the solubility
of different salts in water.
| Soluble salts | Insoluble salts |
| 1. All sodium, potassium and ammonium salts. | silver, mercury(I) and lead chlorides barium, lead (II) and calcium sulphates but other common carbonates are insoluble.but other common hydroxides are insoluble. |
| 2. All nitrates of metals | |
| 3. All chlorides except .………………….. | |
| 4. All sulphates except………………….. | |
| 5. Sodium, potassium, and ammonium carbonates…………………………… hydroxides………………………….…… | |
| 6. Sodium, potassium and ammonium |
methods are available for the preparation of salts. The solubilities of
the prepared salts determine their methods of preparation. Hence, in
the choice of a method of preparation of a particular salt, one has to
be acquainted with its solubility properties.
These methods are sometimes referred to as double decomposition. To
precipitate an insoluble salt, you must mix a solution that contains its
positive ions with the one that contains its negative ions.
Reaction between an acid and an alkali:In
this method, a dilute acid is added to an alkali in the appropriate
volume ratio. The reaction between an acid and an alkali is termed as
neutralization. For example, sodium chloride may be prepared by the
following neutralization reaction:NaOH(aq) + HCl(aq)→ NaCl(aq) + H2O(l). Both
reactants are soluble, and no gas is given off during the reaction. So,
it is difficult to know when the reaction is over. In this case, you
have to use an indicator. A universal indicator or litmus could be used,
but even better is phenolphthalein. This is pink in alkaline solution,
but colourless in neutral or acidic solutions.
Reaction of a metal with an acid:This
is another general method for preparing salts. For example, zinc
sulphate can be made by reacting dilute sulphuric acid with zinc:Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g).
However, this method is not suitable for all metals or all acids. It is
good for preparing salts of fairly reactive metals such as magnesium,
aluminium, zinc and iron. However, the reactions of highly reactive
metals like sodium, potassium and calcium with acids are very violent
and dangerous. The reaction with lead is too slow. Copper, silver and
gold do not react at all.
Reaction of a metal oxide with an acid.Metal
oxides, as you studied early, react with dilute acids to produce salts.
Copper oxide is an insoluble base. Although copper will not react with
dilute sulphuric acid, copper (II) oxide will. The salt that forms is
copper (II) sulphate.CuO(s) + H2SO4(aq) → CuSO4(aq) + H2O(l)
Reaction of a metal carbonate with an acid.The
reaction between metal carbonates and dilute acids are accompanied with
evolution of carbon dioxide gas. The evolution of a gas can be used to
indicate when the reaction is over. An example of such reactions is the
reaction between calcium carbonate and dilute hydrochloric acid. CaCO3(s) + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g)
above methods for preparing soluble salts are specific for each method
mentioned. Generally, soluble salts may be prepared by two broad
methods.
route is essentially the same whether starting with a solid metal, a
solid base (oxide) or a solid carbonate. The route can be divided into
four stages:
Stage 1:An
excess (more than enough) of the solid is added to the acid and allowed
to react. Using an excess of the solid makes sure that all the acid
used up. If it is not used up at this stage, the acid would become more
concentrated when the water is evaporated later (stage 3).
Stage 2:The excess solid is filtered out after the reaction is completed.
Stage 3: The
filtrate is gently evaporated to concentrate the solution. This can be
done on a heated water bath. Do not heat so strongly or “spitting” might
take place.
Stage 4:The concentrated solution is
cooled down to let the crystals form. Filter off the crystals. Wash them
with a little distilled water. Dry the crystals carefully between the
filter papers.
method (titration method) involves the neutralization of an acid with
an alkali (for example sodium hydroxide) or a soluble carbonate (for
example sodium carbonate). Since both the reactants and the products are
colourless, an indicator is used to find the neutralization point or end point (when all the acid has just
been neutralized). Once the end point is reached, the resulting salt
solution is evaporated and cooled to form crystals as described in
method 1.
salts are insoluble in water (for example silver chloride and barium
sulphate – see table 3.6). Such salts are generally prepared by ionic
precipitation.Precipitation is the sudden formation of a solid either:
when two solutions are mixed; or when a gas is bubbled into a solution.
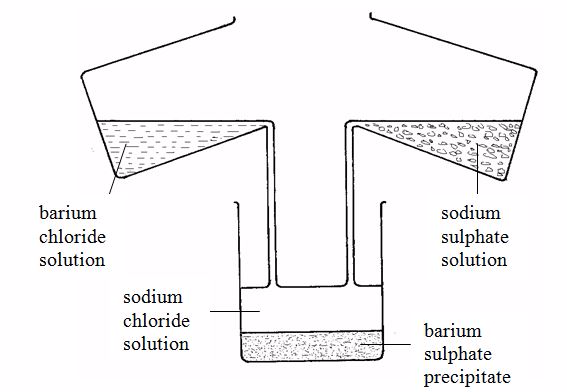
example, barium sulphate can be prepared by adding a solution of a
soluble sulphate (for example sodium sulphate) to a solution of a
soluble barium salt (for example barium chloride). The insoluble barium
sulphate is formed immediately. This solid falls to the bottom of the
container as a precipitate (figure 3.2). The precipitate can be filtered
off. It is then washed with distilled water and dried in a warm oven.
The equation for the reaction is:
shows how important the state symbols can be – it is only through state
symbols that we can tell this equation shows a precipitation.
sulphate could also be made from barium nitrate and sodium sulphate,
for example, since these salts are both soluble. As long as barium and
sulphate ions are present, barium sulphate will be precipitated.
soluble and insoluble salts can be made directly by reacting two
elements together. This is called combination (or synthesis). This type
of reaction is mainly possible for metal chlorides, bromides and
iodides. For instance, if a piece of burning sodium is lowered into a
gas jar of chlorine, the two react violently to produce a white powder
of sodium chloride:
chlorides can also be prepared by combination, for example, iron (III)
chloride and aluminium chloride can be made by heating iron and
aluminium metals in stream of chlorine:
different salts are heated, they behave in different manners. The
crystals of some salts contain water of crystallization. When these
hydrated salts are heated, their water of crystallization is driven off
as steam. The crystals then lose their shape and become a powder. The
following are few examples of hydrated salts:
| Salt formula | Chemical name |
| CuSO4.5H2O | Copper (II) sulphate five water |
| Na2CO3.I0H2O | Sodium carbonate ten water |
| MgCl2.6H2O | Magnesium chloride six water |
| FeCl3.6H2O | Iron (III) chloride six water |
| FeSO4.7H2O | Iron (II) sulphate seven water |
| CoCl2.6H2O | Cobalt (II) chloride six water |
| MgSO4.7H2O | Magnesium sulphate seven water |
| CaSO4.2H2O | Calcium sulphate two water |
of potassium, sodium, calcium, lithium and magnesium are stable to heat
and do not decompose when heated. Other sulphates decompose to give the
oxide and sulphur trioxide gas except iron (III) sulphate which
decomposes to give sulphur dioxide and sulphur trioxide.
that have lost their water of crystallization are called anhydrous. If
water is added back to the anhydrous copper (II) sulphate powder, the
powder turns into blue crystals again and heat is evolved. This can be
used as a qualitative test for water.If the white, anhydrous powder is
further heated strongly, it decomposes to black copper (II) oxide:
iron (II) sulphate is green in colour. When heated, it loses all its
water of crystallization and changes colour from green to white:
chlorides of most metals are hydrated except those of potassium, lead,
mercury and silver. Hydrated chlorides do not usually give the anhydrous
salt when heated. Instead, a chemical change termed as hydrolysis
normally occurs. The reaction is accompanied by the evolution of steam
and hydrogen chloride gas, and the formation of the basic chloride or
oxide. When, for example, hydrated magnesium chloride is heated, its
basic chloride is formed:
same case applies when hydrated calcium chloride is heated. However,
when hydrated aluminum chloride is heated, it does not produce the
anhydrous salt. Instead, the oxide is formed thus:
chloride sublimes when heated. The reaction is reversible and the
products may recombine on cooling to form the salt back.
carbonates of potassium and sodium are very stable to heat. They do not
decompose even when heated to very high temperatures. All other
carbonates decompose when heated to give the oxide and carbon dioxide:
there are very few and exceptional carbonates that do not behave like
this. Ammonium carbonate, for example, decomposes readily when heated to
give ammonia gas, water vapour and carbon dioxide gas:
nitrates of common heavy metals (such as Pb, Al, Ca, Mg, Zn and Cu)
decompose on heating to give the oxide, nitrogen dioxide and oxygen:
and sodium hydroxides are very stable to heat. They do not decompose
even when heated strongly. All other hydroxides decompose to give the
oxide and water vapour, e.g.:
is exposed to air, it absorbs water vapour from the atmosphere and
eventually dissolves. Its tendency to absorb water vapour explains why
it is used as a drying agent for gases (not ammonia, because it combines
with the gas).
sodium hydroxide is also deliquescent. On exposure to air, pellets of
sodium hydroxide quickly become shiny and then sticky as they absorb
water vapour from the atmosphere. Eventually the sodium hydroxide
pellets absorb moisture from the atmosphere so much that they dissolve
to form a solution of sodium hydroxide.
(II) nitrate and zinc chloride are the other deliquescent salts. Pure
table salt (NaCl) is not deliquescent. However, if the salt is directly
obtained from the sea, it is deliquescent. The salt from the sea
contains magnesium chloride as one as the impurities. It is this
magnesium chloride salt that deliquesces and not sodium chloride.
substances tend to absorb water vapour from the air but do not change
their physical states. Copper (II) oxide and calcium oxide are both
hygroscopic solids because they can absorb moisture from the atmosphere
and yet retain their solid states. Because of this behaviour, calcium
oxide is used as a drying agent, which absorbs moisture from gases
prepared in the laboratory.
sulphuric acid is a hygroscopic liquid. When exposed to air, the acid
absorbs water vapour from the atmosphere diluting itself to absorb 3
times its original volume.
may be defined as the tendency of a substance to absorb water vapour
from the atmosphere without changing its physical states.The word
hygroscopy is a general term applied to all substances that absorb water
vapour from the air. Any substance that can take up moisture from the
atmosphere is said to be hygroscopic in nature.
is the tendency of a hydrated substance to lose the water of
crystallization to the atmosphere. Some salt crystals give out some or
all of their water of crystallization to the atmosphere when exposed to
air. Such substances are said to be efflorescent and the process of water loss is known as efflorescence.
Sodium carbonate ten water (washing soda) is a good example of an
efflorescent substance. If washing soda crystals are exposed to open air
at room temperature, they lose some of the water of crystallization.
The solid loses nine of its ten molecules of water of crystallization to
the air. One molecule of water, which remains fixed, can be removed
only by strong heating.
crystal lattice is broken down as the salt loses its after of
crystallization. Thus, transparent crystals of hydrated sodium
carbonates become white and powdery on the surface.
is a wide range of salts. A great number of them play an important role
in our everyday life. The following are the uses of some salts:
chloride often called common salt or table salt, is essential for life
and is an important raw material for industries. At home, it is used for
cooking, that is, flavouring different foods.Biologically, it has a
number of functions: it is involved in muscle contraction; it enables
the conduction of nerve impulses in the nervous system; it regulates
osmosis (the passage of solvent molecules through membranes); and it is
converted into the hydrochloric acid that aids digestions in the
stomach.
industrial uses of sodium chloride include curing bacon, flavouring
foods, and in the manufacture of margarine, butter and cheese. It is
also used to tan leather in the leather industry. Rock salt is used as a
fertilizer for sugar beet, and is spread on roads to melt the ice
during winter. The salt is the starting point for many important
chemicals, for example, the electrolysis of brine (concentrated solution
of sodium chloride) gives sodium hydroxide, chlorine and hydrogen. What
other uses of sodium chloride do you know? Mention them.
An
important use of calcium carbonate is in the building industry. It is
widely used in making cement, lime, mortar and making steel from iron.
Powdered
limestone is used as a liming material to neutralize soil acidity. When
used in this way, it is termed as agricultural lime. When added in the
soil, agricultural lime acts as a calcium source for plants as well as
increasing the pH and water retaining capacity of acidic soils.
It is also used in making paint, plastic, rubber, ceramic and glass; and in oil refining, and iron ore purification.
Calcium carbonate is the most preferred mineral in the paper industry. It helps in the production of the best quality papers.
Since calcium is essential for healthy bones and teeth, it is used as a dietary calcium supplement.
Limestone can also be well shaped, painted, and then used as decorative stones.
CAN, urea, etc are used as nitrogenous fertilizers which are applied to
the soil to improve soil fertility and hence enhance plant growth and
production. Millions of tonnes of fertilizers are produced every year.
Without these chemicals, world food production would probably be halved.
is chiefly used for the manufacture of Plaster of Paris (P.O.P). The
Plaster of Paris is used for making casts for statuary ( the expansion
during setting ensures a fine impression), in surgery to maintain joints
in a fixed position and in cement and wall plasters.
the many other uses of calcium sulphate are as a pigment in white
paints, as a soil conditioner, in Portland cement, as a sizer, filler,
and coating agent in papers, in the manufacture of sulphuric acid and
sulphur, in the metallurgy of zinc ores and as a drying agent in many
laboratory and commercial processes.
carbonate is widely used as one of the raw materials for making glass.
Glass is made by heating a mixture of limestone, sand, sodium carbonate,
and recycled glass in a furnace.The cosmetic industry uses it for
manufacturing soap. The chemical industry uses it as a precursor to
numerous sodium-containing reagents
is also important in photography and in the textile industry. In
addition to these industrial applications, sodium carbonate is used in
medicine as an anti-acid
carbonate has various environmental applications. Large quantities of
the carbonate are used in sewage treatment; in water softening as
washing soda crystals, Na2CO3.10H2O, and in desulphurisation of flue gas.
sulphate is mainly employed for making health salts (laxative, mild
purgative). The health salt is used as a medicine (laxative) which aids
to empty the bowels following constipation or other health problems.
(II) sulphate is used in fungicides, which are sprayed on crops,
especially vines and potatoes, to kill moulds which would injure plants
and hence curtail crop yield. It is also used in the manufacture of
certain green pigments which are used for painting.
It
is largely used in making phosphoric acid and fertilizers. Calcium
phosphate is used in baking. It is also used in cheese products.

















Leave a Reply