STRUCTURE AND PROPERTIES OF MATTER
Structure of Matter
State of matter is defined in terms of the phase transitions which indicate the change in structure and properties. Solids, liquids and gases all are made up of microscopic particles. The behavior of all these particles also varies in three phases.
The Concept of Matter
Explain the concept of matter
Matter is anything, such as a solid, liquid or gas, that has weight (mass) and occupies space. For anything to occupy space, it must have volume.
The Particular Nature of Matte
Justify the particulate nature of matter
Matter is made up of tiny particles. The particles are atom or molecules, examples of substances, which are made up of atoms, are: gold, copper, Argon and silver; and those made up of molecules includes oxygen, water and ammonia.
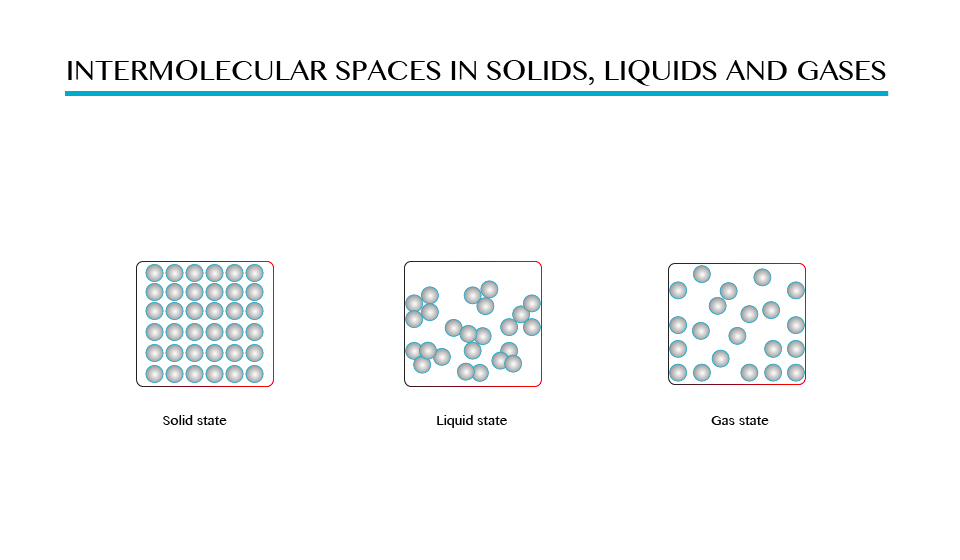
In solid, storm’s attractive forces hold molecules together so that they are not free to move but they can only vibrate about their mean positions.
In liquids there are weak forces of attraction between molecules therefore the molecules are free to move randomly. The distances between molecules in liquids are therefore are larger than in solids.
In case of gases the molecules experience very weak forces of attraction and hence they are free to move randomly filling the whole space of the containing vessel. The distances between molecules in gases are comparatively greater than those in solids and liquids as shown in the figure above.
- Solid state
- Liquid state
- Gaseous state
Solid state is the state of matter, which include solid materials, in which the intermolecular force between molecules are greatest and distance between molecules is small. Examples of solid state are wood, iron, etc.
Gaseous state is the state of matter in which there is no intermolecular forces between molecules hence molecules are free to move from one place to another examples of gases are hydrogen, oxygen, carbon dioxide gas.
Difference between solid state, liquid state and gaseous state of matter
| Solid state | Liquid state | Gaseous state. |
| It concerns with solid matter | It concerns with liquids/ fluids matter | It concerns with gases |
| Have high intermolecular | Low intermolecular force | No intermolecular force |
| No distance between molecules | There is little distance between molecules | Molecules are far from each other |
| Good examples are iron materials, woods etc. | Good examples are water, soda, kerosene and petrol | Good examples are oxygen and hydrogen |
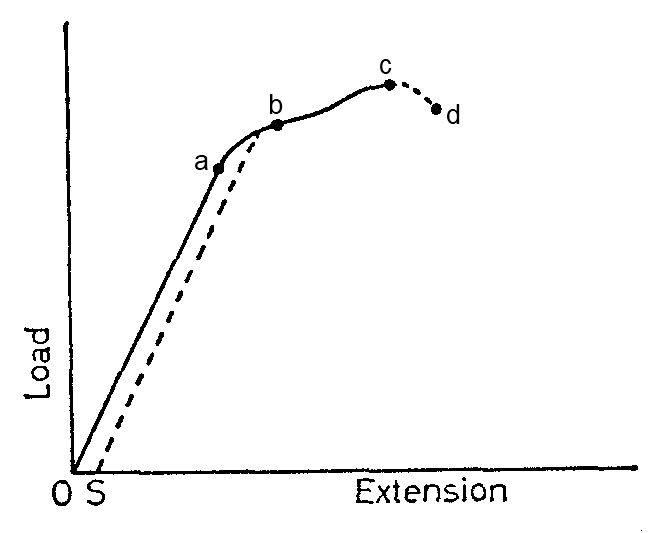
Point A is called the elastic limit. The straight region OA of the graph has a slope K given by the ratio.
The Application of Elasticity in Real Life
Identify the applications of elasticity in real life
In everyday life we often actually do the activities that are concerned with the application of physics. Here are some of the application of physics in everyday life especially in the application of Elasticity:
Spring mattress. When you sit or sleep on a spring mattress, futon style push your weight. Pressured by the compressed spring mattress. Due to the nature of its elasticity, stretch a spring mattress again. Spring will be stretched and compressed, and so on.
Spring that is used as shock absorbers on motorcycles. Springs used in the suspension systems of motor vehicles. The purpose of this is to dampen spring a surprise when a motorcycle driven through an uneven road surface.
Another simple example and that you may often come across is the catapult. When it was about to shoot birds with catapults for example, rubber slingshots first stretch (given the gravity). Due to the nature of its elasticity, long rubber slingshots will return to normal after a tensile force is removed.
Explain the concept adhesion and cohesion
Matter is made up of molecules. That exerts force of attraction. This force of attraction may be either Cohesion or Adhesion.
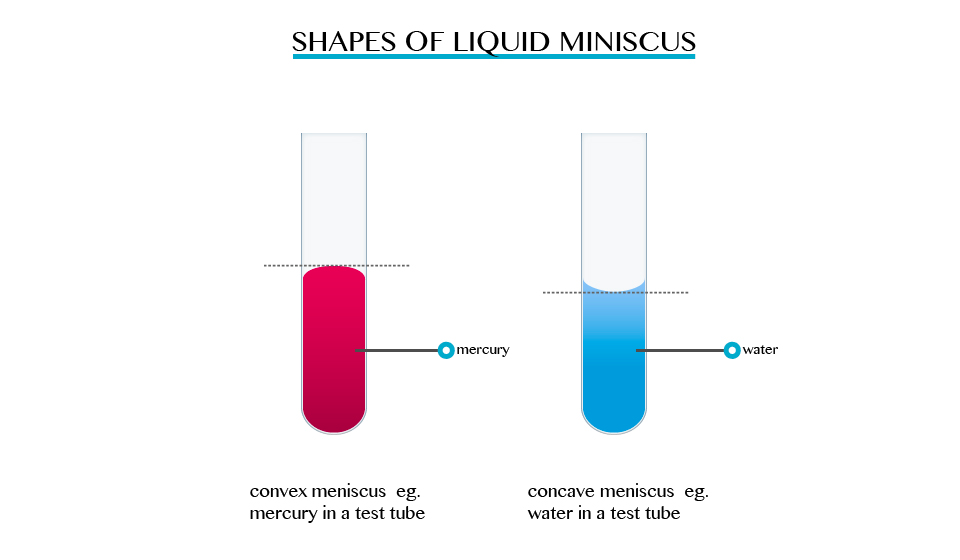
The formation of a meniscus in a liquid is due to forces of adhesion between the liquid and the walls of the container. The adhesion of the liquid such as water to the wall of a vessel causes an upward force on the liquid at the edge.
To stick two different objects together. Here we use the adhesive effects of tape or glue.
Adhesion can also be used to remove harmful materials such as bacteria from drinking water. Adhesive forces are the source attraction substance.
Cohesion assists in transport of water in plants and animals by allowing one molecule to pull others along with it.
The bodies of plants and animals also use the cohesion of tissue to repair damage.
Ink sticks on paper because of adhesive force between the paper and ink.
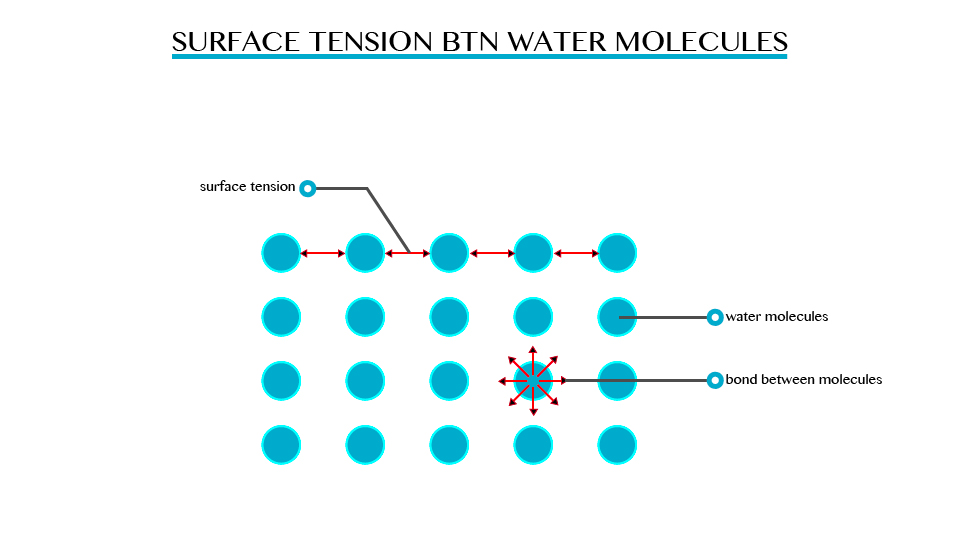
Explain the concept of surface tension
However, when some detergent is added to water, the same objects sink to the bottom of the trough. This means that the detergent interfered with the surface of the liquid so decreasing the tension of the water surface.
Application at surface tension
In extraction of impurities dating laboratory processSurfactants are also used to make emulsion of liquid like oil and water.In cleaning action of soap
Capillarity
Explain the concept of capillarity
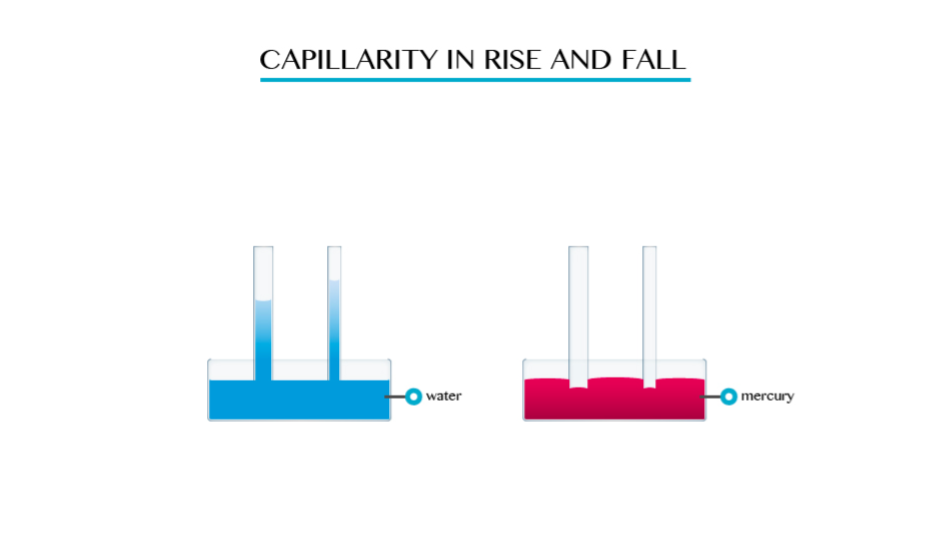
This is the tendency of a liquid to rise in narrow tubes or to be drawn into small openings such as those between the fibres of a towel. Capillarity can pull a column of liquid upward until the weight of liquid becomes greater than the surface tension.
In a tube, capillarity depends on the tube’s diameter but weight of water column depends on other factors besides it.
The smaller the radius of the tube the higher the liquid will rise in it. This implies that capillarity height is immensely proportional to the diameter of the tube.
By definition
Identify the applications of capillarity in daily life
Capillarity is essential to plants and animals.
In plants, it facilitates the transport of water and nutrients from the roots to the leaves where photosynthesis produces the plants food. In animals it assists in the circulation of blood.
Capillarity promotes the movement of ground water.
It is the principles on which paper and fabric towels work to absorb water.
Cotton clothing in hot climates uses capillarity action to draw perspiration away from the body.
In an oil or kerosene lamp capillarity draws the fuel up into the wicker where it can be burnt.
A writing Rubin splits in the middle so that a fine capillary is formed.
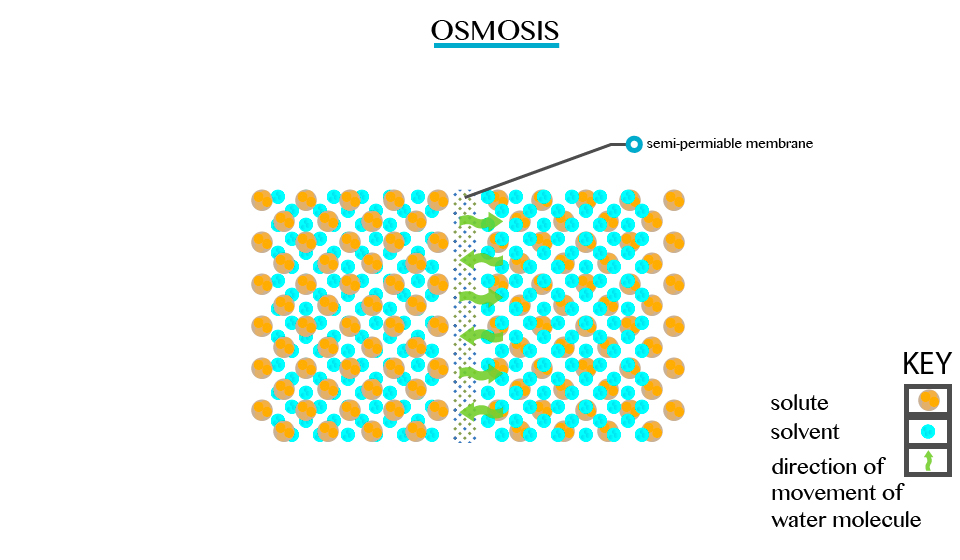
Explain the concept of osmosis
Applications of osmosis in daily life:


















Leave a Reply