Heat sources
Most chemical reactions require heat to proceed. It is therefore important to have sources of heat in a laboratory for heating various reacting substances.
Sources of heat in a chemistry laboratory may include Bunsen
burner, candle, spirit burner, kerosene burner (stove), tin lamp (kibatari) and charcoal burner. These are burners commonly used in most school laboratories.
Different Heat Sources which can be Used in a Chemistry Laboratory
Name different heat sources which can be used in a chemistry laboratory
The
Bunsen burner is the best of all burners because it is convenient to
handle. Another advantage of the Bunsen burner is that it produces a hot
flame whose temperature is approximately 1000°C. The temperature can be adjusted easily to produce a non-luminous flame, which does not produce much soot.
Bunsen burner is the best of all burners because it is convenient to
handle. Another advantage of the Bunsen burner is that it produces a hot
flame whose temperature is approximately 1000°C. The temperature can be adjusted easily to produce a non-luminous flame, which does not produce much soot.
Spirit burner
The spirit burner can also produce a soot-free flame. But the flame is not
hot enough to effect (produce) some chemical reactions. Apart from that,
the burner is filled with spirit, a substance that is highly flammable.
hot enough to effect (produce) some chemical reactions. Apart from that,
the burner is filled with spirit, a substance that is highly flammable.
Spirit lamp
A candle
A
candle can only be used where a chemical reaction does not require much
heat. Its disadvantage is that it produces a lot of soot. The other
burners, though not commonly used, are an electric heater and a gas
burner.
candle can only be used where a chemical reaction does not require much
heat. Its disadvantage is that it produces a lot of soot. The other
burners, though not commonly used, are an electric heater and a gas
burner.
The
electric heater uses electricity. The gas burner uses a liquefied gas.
The disadvantage of an electric burner is that it cannot be used in
rural areas where there is no electricity.
electric heater uses electricity. The gas burner uses a liquefied gas.
The disadvantage of an electric burner is that it cannot be used in
rural areas where there is no electricity.
Candle
A kerosene burner
A kerosene burner (stove), also called jiko la mchina
in Swahili, if well adjusted can produce a flame hot enough to heat
many substances in the laboratory. It is fulled with kerosene, a fuel
that is convenient to carry and store. This fuel does not catch fire
easily as compared to spirit and it is affordable
in Swahili, if well adjusted can produce a flame hot enough to heat
many substances in the laboratory. It is fulled with kerosene, a fuel
that is convenient to carry and store. This fuel does not catch fire
easily as compared to spirit and it is affordable
It
can conveniently be used by schools in the most remote areas where
there is no electricity. If too much heating is required, wire gauze
should be placed on top of the burner. This will enable reduce soot and
increase the heating temperatures to about 1000°C or more.
can conveniently be used by schools in the most remote areas where
there is no electricity. If too much heating is required, wire gauze
should be placed on top of the burner. This will enable reduce soot and
increase the heating temperatures to about 1000°C or more.

Kerosene burner (stove)
A charcoal burner
A
charcoal burner can also be used in remove areas. In case the kerosene
burner is not available, for one reason or another, a charcoal burner
can be the best alternative.
charcoal burner can also be used in remove areas. In case the kerosene
burner is not available, for one reason or another, a charcoal burner
can be the best alternative.
The red-hot charcoal on the burner is almost soot-free. It can produce high temperature sufficient to carry out many reactions.

Charcoal burner
A tin lamp
A tin lamp (kibatari), though it produces a lot of soot, can also be used as a burner in a laboratory, especially in remote areas.
However, the heat it produces is not hot enough to initiate some reactions.
Tin lamp
The Functioning of a Bunsen Burner
Explain the functioning of a bunsen burner
Of
all the burners we have discussed so far, a Bunsen burner is the mostly
used. Therefore, we are going to discuss about the functioning of the
Bunsen burner in more detail. As the name suggests, this burner was
invented by a German scientist called Robert Bunsen, so it was named
after his name as a Bunsen burner. The burner uses coal gas, which burns
with a hot and non-luminous flame when the air holes are open. This is a
kind of flame we normally use in the laboratory.
all the burners we have discussed so far, a Bunsen burner is the mostly
used. Therefore, we are going to discuss about the functioning of the
Bunsen burner in more detail. As the name suggests, this burner was
invented by a German scientist called Robert Bunsen, so it was named
after his name as a Bunsen burner. The burner uses coal gas, which burns
with a hot and non-luminous flame when the air holes are open. This is a
kind of flame we normally use in the laboratory.
Functions of different parts of the Bunsen burner
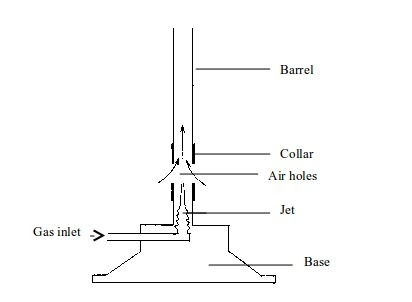
Base: Supports the burner. It makes the burner stable, due to its heavy weight, when placed on a bench.
Gas inlet: Lets the gas in from the gas supply.
Jet: Directs the gas to the barrel
Collar:
Regulates the amount of air entering the burner. It has air holes that
can be turned open or closed depending on the kind of flame, and hence
amount of heating required.
Regulates the amount of air entering the burner. It has air holes that
can be turned open or closed depending on the kind of flame, and hence
amount of heating required.
Air holes: These small holes on the collar allow air to enter in the burner.
Barrel: This is a part of the burner where air (from outside), and gas (from gas supply) mix up and burn.
How to light a Bunsen burner
After
knowing the different parts of the Bunsen burner, it is important that
you also learn how to light it. This is because careless use of the
burner may lead to accident or wastage of the gas. The following is a
correct sequence of steps on how to light the Bunsen burner:
knowing the different parts of the Bunsen burner, it is important that
you also learn how to light it. This is because careless use of the
burner may lead to accident or wastage of the gas. The following is a
correct sequence of steps on how to light the Bunsen burner:
- Connect the Bunsen burner by a rubber tube to the gas supply.
- Close the air holes.
- Turn the gas tap on to let in sufficient gas.
- Quickly bring a flame at the top of the barrel. You may use a matchstick, a lighter or wooden splint as a source of flame.
- Turn the collar to adjust the air holes until you get the type of flame you want. You may have the holes completely open.
- Adjust the gas tap until the gas supply is enough to produce a non-luminous flame.
To
put off the flame of the burner after you finish heating a substance,
turn the gas tap off in order to cut off the gas supply to the burner.
Disconnect the burner from the gas mains by removing the rubber tube
connecting the two. Then close the air holes. Pay attention not to touch
the hot collar with your fingers or else wait until it is cool enough.
Take the Bunsen burner and keep it at the appropriate place
put off the flame of the burner after you finish heating a substance,
turn the gas tap off in order to cut off the gas supply to the burner.
Disconnect the burner from the gas mains by removing the rubber tube
connecting the two. Then close the air holes. Pay attention not to touch
the hot collar with your fingers or else wait until it is cool enough.
Take the Bunsen burner and keep it at the appropriate place
Types of flame
Flames
are formed by burning gases or vapours. During burning, heat and light
are given out. For any solid or liquid to burn with a flame, it must
first turn into inflammable vapours (gaseous state).
are formed by burning gases or vapours. During burning, heat and light
are given out. For any solid or liquid to burn with a flame, it must
first turn into inflammable vapours (gaseous state).
Luminous and Non-luminous Flames from Different Types of Flames
Produce luminous and non-luminous flames from different types of flames
A
flame can be luminous or non-luminous. Flames of a candle and any oil
are usually smoky and luminous. Flames of such kind are normally of
little laboratory use. This is because they are not hot enough and would
deposit soot on laboratory apparatus. Coal gas also burns with a smoky
and luminous flame. With a Bunsen burner, one can produce two types of
flames namely, the luminous and non-luminous flames.
flame can be luminous or non-luminous. Flames of a candle and any oil
are usually smoky and luminous. Flames of such kind are normally of
little laboratory use. This is because they are not hot enough and would
deposit soot on laboratory apparatus. Coal gas also burns with a smoky
and luminous flame. With a Bunsen burner, one can produce two types of
flames namely, the luminous and non-luminous flames.
Luminous flame
This
is a type of flame produced when the air holes of a Bunsen burner are
closed. When the air holes are closed very little air enters the barrel
of the burner. In this case, the flame will be large, unsteady and
bright
is a type of flame produced when the air holes of a Bunsen burner are
closed. When the air holes are closed very little air enters the barrel
of the burner. In this case, the flame will be large, unsteady and
bright
The flame will have four main zones each having a distinct colour.
- The inner dark zone – This is dark, cool and contains unburnt gas
- Luminous yellow zone –
The gas burns in this zone but because the air is not enough the
burning is incomplete. This leads to formation of tiny carbon particles
from the gas. When these particles are white-hot, they result in
formation of light (the yellow colour we see). If a cold evaporating
dish, porcelain crucible, or glass is placed in this zone, it will
blacken due to deposition of carbon particles (soot) on it. - Outer zone –
This is a non-luminous zone where the burning of the gas is complete
due to presence of enough air. Because of the absence of carbon
particles, this zone does not give out light. Consequently, the zone
cannot be seen easily. - Blue zone – Due to rising convectional current, there is sufficient supply of air for complete burning at this zone.
Non-luminous flame
When
air holes are fully opened, sufficient air enters the Bunsen burner
barrel and mixes well with the coal gas. Hence, the burning of the gas
is much quicker and complete. The flame is smaller and hotter.
air holes are fully opened, sufficient air enters the Bunsen burner
barrel and mixes well with the coal gas. Hence, the burning of the gas
is much quicker and complete. The flame is smaller and hotter.
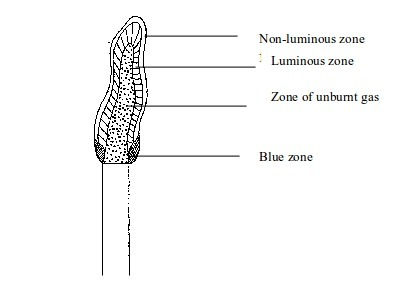
Due
to absence of white-hot carbon, no light appears. The flame is
therefore non-luminous. The flame has three district zones each with a
different colour.
to absence of white-hot carbon, no light appears. The flame is
therefore non-luminous. The flame has three district zones each with a
different colour.
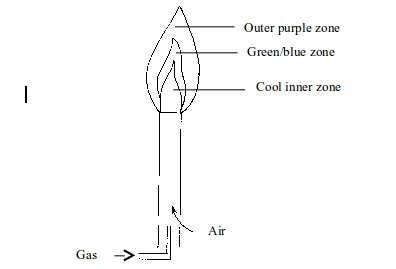
Non–luminous flame
- Cool inner zone – this is a zone of unburnt gas.
- Green/blue zone –
part of the gas burns in this zone because there is not enough air to
burn all the gas completely. However, no carbon is formed. The hottest
part of the flame is at the tip of this zone. - Outer purple zone – Burning of the gas in this zone is complete.
Major differences between luminous and non-luminous flames
| Non luminous flame | Luminous flame | |
| 1. | Formed when air holes are open | Formed when air holes are closed |
| 2. | Very noisy | Silent or calm |
| 3. | Comprises of three zones | Comprises of four zones |
| 4. | Forms no smoke or soot on apparatus | Forms a lot of smoke or soot on apparatus |
| 5. | Blue and almost invisible | Bright yellow and clearly visible |
| 6. | Very hot flame | Not a hot flame |
| 7. | Not bright | Very bright |
| 8. | Triangular flame | Wave-like flame |
Investigation of different parts of a flame
We can easily find out whether or not the inside of a flame is cool. Two experiments can prove this:
- (a) When a piece of cardboard is held horizontally over a non-luminous flame, we notice a burn mark as shown below:
When
held vertically over the flame, the burn mark is as shown in above.
Note that when performing this experiment, the cardboard should be
withdrawn from the flame just before it catches fire. We find that the
middle part of the cardboard does not get burned. This is the part in
the zone containing unburnt gas.
held vertically over the flame, the burn mark is as shown in above.
Note that when performing this experiment, the cardboard should be
withdrawn from the flame just before it catches fire. We find that the
middle part of the cardboard does not get burned. This is the part in
the zone containing unburnt gas.
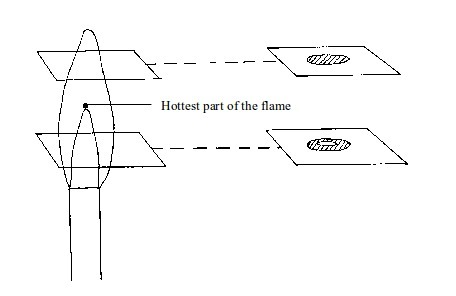
Burn mark on cardboard when held horizontally
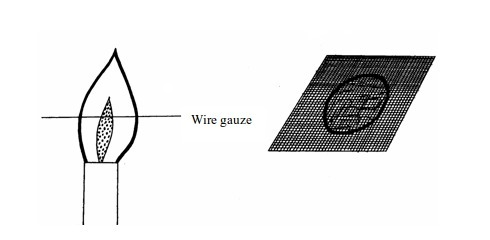
(b)
If the above experiment is repeated using a wire gauze, we notice that
the part in the middle will not become red hot except when the gauze is
held in the flame for a long time.
If the above experiment is repeated using a wire gauze, we notice that
the part in the middle will not become red hot except when the gauze is
held in the flame for a long time.
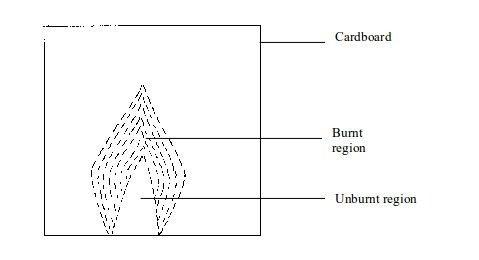
Burn mark on cardboard when held vertically
We
can prove the presence of unburnt gas in the Bunsen flame. This can be
done by inserting a glass tube into the flame as shown in figure bellow
can prove the presence of unburnt gas in the Bunsen flame. This can be
done by inserting a glass tube into the flame as shown in figure bellow
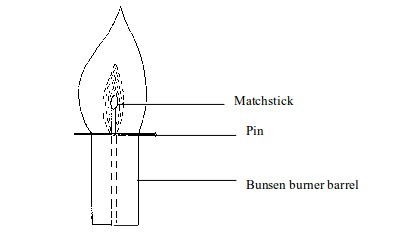
The
unburnt gas can be shown to have risen up the tube by putting a light
at the top of the tube. The flame will form. This indicates the escape
of unburnt gas through the tube.
unburnt gas can be shown to have risen up the tube by putting a light
at the top of the tube. The flame will form. This indicates the escape
of unburnt gas through the tube.
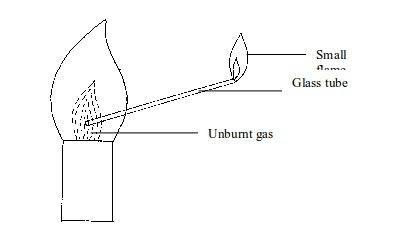
To indicate the presence of unburnt gas in a Bunsen burner flame
Uses of flames
Flames are used for different purposes. Some uses of the flames include the following:
- Production of heat for heating substances in the laboratory:
In this case, a non-luminous flame, which produces much heat, is used.
However, for reactions that require little heat, a luminous flame, which
is not very hot, can be used. - Flame tests for elements:
In chemical analysis of some elements, a flame test is one of the
preliminary tests normally used to identify an element. When some
elements are strongly heated, they produce characteristic flame colours
that distinguish them from one another. A non-luminous flame is often
used. - Production of light: Flames produce light that
can be used to light a dark room. Therefore, an experiment that involves
heating can even be conducted in the dark. The same flame is used to
give heat as well as light. Here, a luminous flame is used. Examples of
heat sources, which produce flames that may be used for lighting, are
hurricane lamp, tin lamp, spirit lamp and candle. - Cooking:
Since it gives a hot flame and produces no soot, a non-luminous flame
can be used for cooking food. Gas cookers, gas stoves and kerosene
stoves usually produce such flames. - Welding: A
non-luminous flame is suitable for welding because it is very hot. In
most welding operations, an oxyacetylene gas, a mixture of oxygen and
ethyne, is used. When burned, the gas produces a flame hot enough to cut
or melt the metal.

















Enjoyed every bit of your blog article.Really looking forward to read more. Cool.