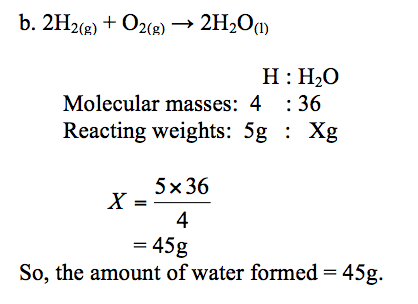TOPIC 1: CHEMICAL EQUATIONS | CHEMISTRY FORM 3
A chemical equation is a representation of a chemical reaction with the help of symbols and formulae of the substances involved in the reaction.
It is a chemical shorthand for representing the reacting substance or substances combining (the reactants) and the substance or substances formed as a result of the reaction (the products).
Molecular Equations
A Molecular equation is the one which shows the reactants combining and the products formed, in their elemental or molecular forms in a chemical reaction. An example of a molecular equation is the reaction between sodium and water to produce sodium hydroxide solution and hydrogen gas:
2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g)
In this context, sodium (in elemental form) reacts with water (in molecular form) to produce sodium hydroxide (in molecular form) and hydrogen gas (in molecular form).
Word Equations for given Chemical Reactions
Write word equations for given chemical reactions
A word equation is a short form of expressing a chemical reaction by word. Chemical reactions can be summarized by word equations that show all the reactants and the products. This type of equation links together the names of the reactants and the products. For examples, the burning of magnesium in air to produce magnesium oxide can be represented by the following word equation:
Magnesium + Oxygen → Magnesium oxide
Another example is the reaction between sodium and chlorine to give sodium chloride (common salt)
Sodium + Chlorine → Sodium chloride
Equations like these sometimes give us some information about the products formed
when different substances are reacted together. But equations can be
made even more useful by writing them using chemical symbols and
formulae.
Any method for representing a chemical reaction must meet basic certain requirements. These are:
the
chemical nature of the reactants as well as those of the products must
be clear. The reactants can be in solid, gaseous, liquid or aqueous
forms.
the mole ratios in which the products are combined and
the products are formed must be deducible. This means that atoms of the
reactants and the products must be balanced.
the direction of
the reaction must be established. This means that it should be clearly
shown which substances are the reactants and which ones are the
products. This is normally done by separating the reactants from the
products by an arrow. The arrow normally points from the reactants to
the products.
Consider the reaction between potassium and water:
2K(s) + 2H2O (l) → 2KOH (aq) + H2 (g).
In this reaction, the three requirements have been met:
The
chemical nature of the reactants [potassium (solid); water (liquid)]
and the products [potassium hydroxide (aqueous); hydrogen (gas)] has
been shown.
The mole ratios of the reactants and products are
clearly shown: 2 moles of potassium combines with 2 moles of2water to
produce 2 moles of potassium hydroxide and one mole of hydrogen gas.
The
reactants (potassium and water) and the products (potassium hydroxide
and hydrogen) are separated by an arrow (→) which also indicates the
direction of the reaction.
Formula Equations Using Chemical Symbols
Write formula equations using chemical symbols
Essentially,
chemical reactions can be expressed in two forms. The chemical reaction
can be expressed either as a word equation or as a formula (or
symbolic) equation. We have already seen how chemical equations can be
represented by words (word equation). The formula equation makes use of
chemical symbols and formulae to represent a chemical reaction. An
example is the reaction between iron and sulphur to form iron (II)
sulphide: Fe + S → FeS
Balancing Chemical Equations
Balance chemical equations
A
balanced chemical equation has an equal number of atoms of different
elements of the reactants and the products on both sides of the
equation. A balanced equation gives us more information about a reaction
than we get from a simple word equation.
Below is a step-by-step approach to working out the balanced equation for the reaction:
Write the chemical equation for the reaction with the correct symbols and formulae of the reactant(s) and the product(s).
Identify different atoms of the different elements of the reactant(s) and the product(s).
Check whether these different atoms are equal on both sides of the equation. Some atoms may balance each other directly.
Balance the atoms on each sides of the equation by Hit and Trial Method.
Add state symbols.
Example 1
The reaction between hydrogen and oxygen to produce water:
Hydrogen + Oxygen → Water
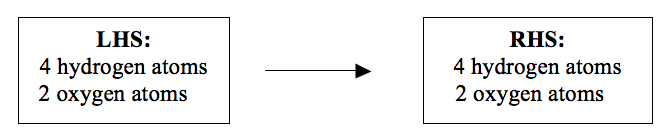
H2 + O2 → H2O (not balanced)
The
atoms involved in the reaction are hydrogen and oxygen. It is these
atoms that we are going to balance. The atoms must be equal on both
sides of the reaction equation. There are two hydrogen atoms on each
side of the equation. But, as you can see there are two oxygen atoms on
the left-hand side (LHS) of the equation and only one oxygen atom on the
right-hand side (RHS). To balance oxygen atoms, we write 2 before
water.
H2 + O2 → 2H2O (not balanced yet)
By
introducing 2 before water, another problem has been created. Now we
have 4 hydrogen atoms on the RHS but only 2 hydrogen atoms on the LHS.
To equalize the number of hydrogen atoms we write 2 before hydrogen on
the LHS.
2H2 + O2 → 2H2O (balanced).
You
can still check to find out whether the atoms are balanced or not. Now
look at the number of atoms on each side of the equation:
Now,
the number of hydrogen and oxygen atoms is the same on both sides of
the equation. This is because the atoms do not disappear during a
reaction. They are neither created nor destroyed. They obey the Law of
Conservation of Mass. When the numbers of different atoms are the same
on the both sides, an equation is said to be balanced. Once the equation
is balanced you can now add the state symbols.
This gives a standard and an acceptable chemical equation.
An
equation which is not balanced is not correct. An unbalanced equation
implies that the atoms have been created or destroyed. It is therefore,
wrong and calculations based on it are certainly unreliable.
Remember
that we cannot change the formulae of the substances involved in the
reaction. These are fixed by the bonding in the substance itself. For
instance, in attempt to balance the number of oxygen in water, H2O, we cannot write H2O2. We can only put a multiplying numbers before symbols and formulae, e.g. 2H2O.
Example 2
Hydrogen burns in oxygen to form water. The equation for the reaction is:
2H2(g) + O2(g) →2H2O(l)
How much oxygen is needed to burn 1g of hydrogen?
How much water is formed when 5g of hydrogen is completely burned in oxygen? (Atomic weights: H = 1, O = 16)
a. Reaction equation:2H2(g)+ O2(g)→2H2O(l)
Molecular weights: 4 : 32
Reacting weights: 1g : Xg
The weight, X, of oxygen = 1×32⁄4= 8g
So, 1g of hydrogen needs 8g of oxygen
Ionic Equations
The Different Between Molecular Equations and Ionic Equations
Differentiate between molecular equations and ionic equations
Ionic
equations are equations in which the reacting substances are
represented in ionic forms after the elimination of spectator ions. In
other words, ionic equations are those equations represented in such a
way that spectator ions are not included in the final equation.
Spectator ions refer to those ions, which do not change during the
reaction i.e. they do not take part in a chemical reaction.
In
order to be able to derive an ionic equation from a molecular equation,
one must be acquainted with the solubility rules as outlined below:
All sodium, potassium and ammonium salts are soluble.
All nitrates, chlorates and acetates are soluble.
All
binary compounds of the halogens (other than F) with metals are
soluble, except those of silver, copper, lead and mercury (lead halides
are soluble in hot water).
All sulphates are soluble except those of silver, lead, mercury (I), barium, strontium and calcium.
All carbonates, sulphites and phosphates are insoluble except those of ammonium and alkali metal (Group I) cations.
All hydroxides are insoluble except those of ammonium, barium and alkali metal (Group I) cations.
All sulphides are insoluble except those of ammonium, alkali metal (Group I) cations and alkali earth metal (Group II) cations.
All
oxides are insoluble except those of calcium, barium and alkali metal
(Group 1) cations; these soluble ones actually react with the water
(hydrolyse) to form hydroxides.
Balanced Ionic Equations
Write balanced ionic equations
Example 3
Consider the reaction for neutralization of hydrochloric acid with sodium hydroxide.
Step 1: HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
Step 2:H+(aq)+Cl–(aq)+Na+(aq)+OH–(aq)→ Na+(aq)+Cl–(aq) + H2O(l)
Step 3: :H+(aq)+Cl–(aq)+Na+(aq)+OH– (aq) → Na+(aq)+Cl–(aq) + H2O(l)
Step 4: H+(aq)+ OH–(aq) → H2O(l)
Post Views: 96