HYDROGEN
Hydrogen is the lightest of all the elements. There is very little hydrogen in the earth’s atmosphere. Hydrogen is so light that its molecules are not held by the earth’s gravity and they diffuse into space.
Overall, it is the most common element in the universe. It is probable that is forms about 90% of the total mass of the universe. It is believed that the sun composes almost of hydrogen and helium.
Hydrogen occurs naturally in air as hydrogen gas. It also occurs in combined state in water, acids, petroleum, and natural gas and in almost all organic substances (proteins, carbohydrates, fats, etc.).
Preparation and Properties of Hydrogen
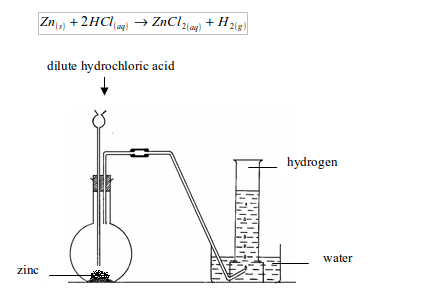
The Preparation of Hydrogen Gas in a Laboratory
Explain the preparation of hydrogen gas in a laboratory
The gas may be collected by downward displacement of water. But when the gas is required free from moisture it is passed through water to remove first, any hydrogen chloride gas and then through concentrated sulphuric acid to remove moisture before being collected by upward delivery. The gas is prepared by upward delivery method because it is lighter than air and is soluble in water.
The Properties of Hydrogen
Includes
- It is a colourless, tasteless and odourless gas.
- It is almost insoluble in water (2 volumes of hydrogen gas dissolve in 100 volumes of water at 8ºC).
- It is the lightest of all gases. It is about 20 times lighter than air (one litre of hydrogen at 0ºC and 760 mmHg pressure weighs 0.0899 grams)
- It condenses at -254ºC to a colourless liquid (and liquid hydrogen freezes at -259 ºC to form colourless crystals).
- It is neutral to litmus. 6. It does not support combustion.
Chemical properties
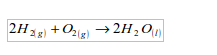
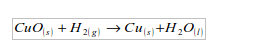
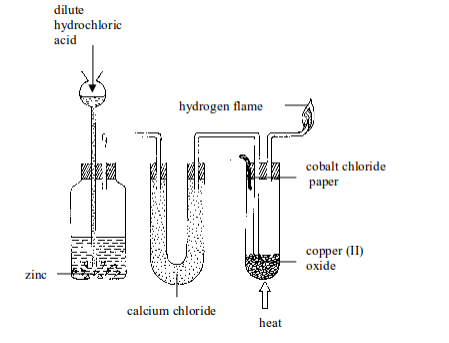
An experiment on reduction of copper (II) oxide (CuO) using hydrogen
- Put about 5 g of copper (II) oxide in a Pyrex test tube and set up the apparatus as shown in figure bellow. Observe and note the colour of copper (II) oxide before the start of the experiment. What colour is it?
- By means of a thistle funnel, add hydrochloric acid in a bottle containing zinc metal to generate hydrogen gas. Pass the gas through a U-tube containing a solid drying agent, calcium chloride.
- Place a dry cobalt (II) chloride paper near the mouth of a test tube as shown in figure bellow.
- Allow the hydrogen gas to pass through the apparatus for some time in order to displace all the air before lighting it.
- Heat the copper (II) oxide strongly until no further changes in colour of the cobalt (II) chloride paper takes place. You may repeat the experiment using lead (II) oxide and compare the results.
- What happens to the copper (II) oxide during the experiment?
- (a) What happens to cobalt (II) chloride paper? (b)Why is it used? (c) What other substance can serve the same purpose as cobalt (II) chloride paper?
- Enough time should be allowed for all the air in the test tube to be replaced by hydrogen before lighting the gas. What is bad about lighting a mixture of air and hydrogen?
- What do you think can cause the size of the hydrogen flame to deteriorate?
- (a) What element did hydrogen take from the copper (II) oxide? (b) Can hydrogen take the same element from any metal oxide?
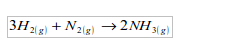


















Leave a Reply